DOMPERIDONE
Product Details:
- Shelf Life 3 Years
- Melting Point 242.5 C
- Loss on Drying 0.14% to 2.06%
- Solubility practically insoluble in water
- EINECS No 260-968-7
- Molecular Weight 425.91 g/mol Grams (g)
- Storage Room Temperature
- Click to View more
DOMPERIDONE Price And Quantity
- 5 Kilograms
DOMPERIDONE Product Specifications
- practically insoluble in water
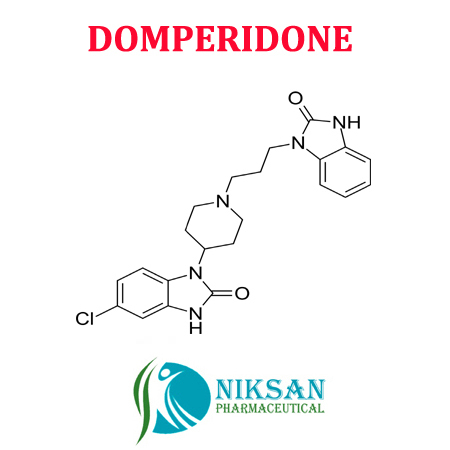
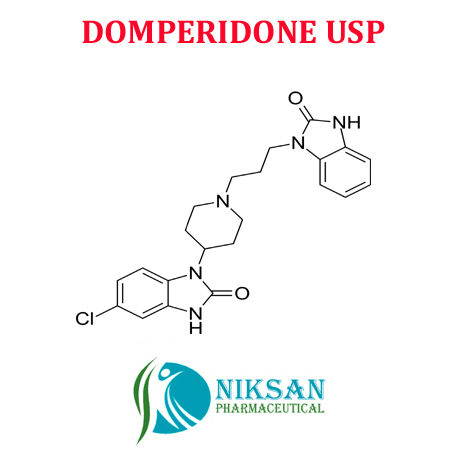
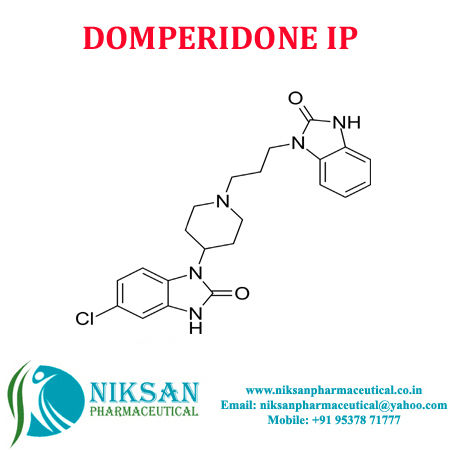
- 5-Chloro-1-[1-[3-(2,3-dihydro-2-oxo-1H-benzimidazol-1-yl)propyl]piperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one
- 425.91 g/mol Grams (g)
- C22H24ClN5O2
- 10ppm
- 260-968-7
- 0.14% to 2.06%
- 723.6C
- DOMPERIDONE
- White to Off-White Solid
- 242.5 C
- 3 Years
- 57808-66-9
- Powder
- Room Temperature
- Medicine Grade
- 99 %
- 10 to 50 microns
- C22H24ClN5O2
- pH 7
- 29420090
DOMPERIDONE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
is words top leading manufacturer, exporter, trader and supplier of DonepezilAPI as well as finished pharmaceutical products of Donepezilamong the pharmaceutical companies. Our product Donepezilis widely used and appreciated by our group companies and also ourcustomers and users all around the nations. We offer the Donepezilin very affordable price.
NiksanPharmaceutical provides API and finished formulations of Donepezil in all over Indian states Like Kerala, Gujarat, Haryana,Rajasthan, Madhya Pradesh, Uttar Pradesh, Rajasthan, Karnataka, Meghalaya,Tamilnadu, Goa, Sikkim, Assam, Punjab, Delhi, Bihar, Jammu Kashmir Etc.
Niksan Pharmaceuticalis also large exporters of the API and finished pharmaceuticalproducts of Donepezil in manycountries for years. The countries where we exporting are Puerto Rico, UnitedStates, Finland, Ireland, New Zealand, Canada, United Kingdom, Belgium, Sweden,Australia , South Korea Austria, Hong Kong, Singapore, Germany, Philippines, Israel,Italy, Romania, Nigeria, Czechia, Denmark, Switzerland, Norway , Croatia,Greece, Taiwan, Malaysia, United Arab Emirates , Portugal, Poland, Thailand,Indonesia, Egypt , Iran, Hungary, Brazil, Saudi Arabia, Pakistan , SouthAfrica, France, Turkey, China, Vietnam , Netherlands, Mexico, Colombia, Spain,Japan and many more countries.
Donepezil is the medication used totreat the Alzheimer disease. Alzheimer disease is the condition which causesbrain cells to die or waste away and by this Alzheimers diseasecan cause mental disorders and loss of memory. Donepezil belongs to the cholinesterase inhibitorsclass of medicine.
SYNONYMS: Domepezil, Donepezil, Donepezilo,Donepezilum.
IUPAC NAME OF DONEPEZIL:
CAS NO: 120014-06-4
FORMULA: C24H29NO3
MOLECULAR MASS: 379.49 g/mol
STORAGE CONDITIONS: Store in cool and dry place away from themoisture and sun light. Do not place it in bathroom or humid place. Keep awayfrom children and pets.
HOW TO USE DONEPEZIL: Do not crush, chew or spit the medication.
HOW DONEPEZILWORKS: Donepezilused in the Alzheimer disease but Donepezil does not cure the disease it isonly improve the memory, awareness, and treat confusion. Donepezil inhibits theacetyl cholinesterase enzyme activity and by this process improves the mentalcondition and brain work.
PHARMACOKINETICS OF DONEPEZIL: Donepezil absorbed slowly by the GI trackafter the oral administration. Donepezil have 100% bioavaibility and reaches Tmax in 3-4 hours. Almost 96% of Donepezil binds with the plasmaprotein in the body. The half-life of Donepezil is very high. Donepezil hashalf-life of 70 hours. Almost 57% of Donepezil eliminated through the urinationand only 5% Donepezil eliminated in feces.
SIDE EFFECTS: The common side effects like nausea,vomiting, tiredness, shakiness, muscle cramps, weakness, trouble in sleeping,dizziness are seen in the patients. Tell your doctor if you have effects likeblack stool, vomit that look like coffee, stomach or abdominal pain, trouble inurination. The allergic reaction is very rare like rash, itching, swelling oftongue, face, trouble in breathing.
PRECAUTIONS: Tell your doctor your medical history if youhave intestinal disease like bleeding, trouble in urination, asthma, COPD<fainting, stomach disease etc. Do not take medicine if you are pregnant or inlactation period. This drug has dizziness effect so do not consume alcohol ormarijuana while completing the dose. Donepezil cause heart problems so kindlytake your doctors advice.
CDSCO APPROVAL: Donepezil (for additional indication)approved by CDSCO in India in 10.08.2005, Donepezil HCl. approved by CDSCO inIndia in 22.03.2001,
Donepezil Hydrochloride Orodispersible Tablet 5mg/10mg approved byCDSCO in India in 15.10.2009,
Memantine 5mg /10mg + Donepezil 5mg Tablets approved by CDSCO inIndia in 31.01.2008,
Donepezil Sustained Release Tablet 23mg (Modified Dosage Form)03.06.2011.
FORMULATION AVAILABLE IN MARKET:
Donepezil 5mg tablets
Donepezil 10mg tablets
Donepezil 23mg tablets
Donepezil 5mg + Memantine 5mg tablets
Donepezil 5mg + Memantine 10mg tablets
Donepezil Hydrochloride Orodispersible Tablets 5mg
Donepezil Hydrochloride Orodispersible Tablets10mg
Donepezil HCl tablet
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Advanced Support for Digestion and Nausea Relief
Domperidone simplifies digestion by enhancing gastrointestinal motility and effectively preventing nausea and vomiting. Its unique mechanism targets D2 receptors, making it a valuable ingredient in medicines for digestive support and nausea control. Frequently recommended in both hospital and clinical settings, this raw material enables the creation of high-performance treatments for related digestive disturbances.
Optimal Storage for Lasting Quality
Ensure Domperidones efficacy and longevity by storing it in a cool, dry area at room temperature, away from direct sunlight and moisture. Do not place this material in bathrooms or humid environments, as this might compromise its quality and reduce shelf life. With proper care, Domperidone retains its stability and effectiveness for up to three years.
FAQs of DOMPERIDONE:
Q: How should Domperidone be administered for optimal results?
A: Domperidone should be taken by mouth, ideally 30 minutes before meals and at bedtime. Always follow your healthcare providers instructions on dosage, and avoid exceeding the recommended amount to prevent potential side effects.Q: What is the primary function of Domperidone in medical formulations?
A: Domperidone is mainly used as an antiemetic agent to prevent nausea and vomiting. It also aids digestion by promoting gastrointestinal motility, and can be used as a galactagogue for increasing milk production.Q: When is the best time to use Domperidone?
A: The best times to use Domperidone are 30 minutes before meals and at bedtime, as directed by your physician. This timing maximizes its effectiveness in easing digestive discomfort and controlling nausea.Q: Where should Domperidone be stored to maintain its stability?
A: Store Domperidone at room temperature in a dry place, away from direct sunlight. Avoid keeping it in the bathroom or any humid area to preserve its quality for up to three years.Q: What is the manufacturing process for Domperidone raw powder?
A: Domperidone powder is synthesized according to strict pharmaceutical standards, ensuring high purity (99%) and consistent particle size (10 to 50 microns). It is distributed, exported, and supplied by trusted pharmaceutical companies in India.Q: What are the benefits of using Domperidone?
A: Domperidone helps prevent and treat nausea and vomiting, promotes better digestion, and may stimulate milk production for mothers needing galactagogue support. Its proven efficacy supports a variety of therapeutic and clinical applications.Q: What precautions should be taken while using Domperidone?
A: Do not exceed the prescribed dose, as overdosing can lead to side effects. Always use this medicine under healthcare supervision and ensure correct storage conditions to maintain its potency.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese