CILOSTAZOL IP & CILOSTAZOL USP
Product Details:
- Boiling point 664.7 C
- Molecular Formula C20H27N5O2
- Heavy Metal (%) 0.5 %
- Particle Size 10 MICRON
- Melting Point 159.4-160.3
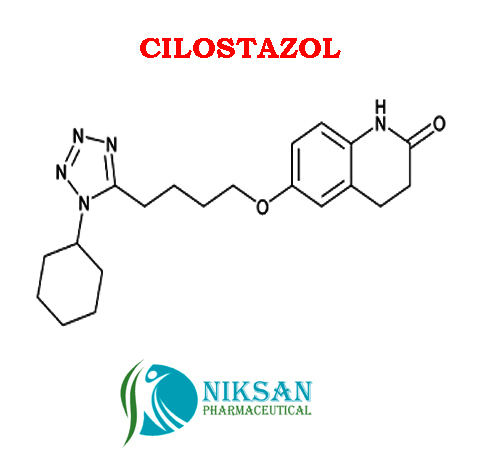
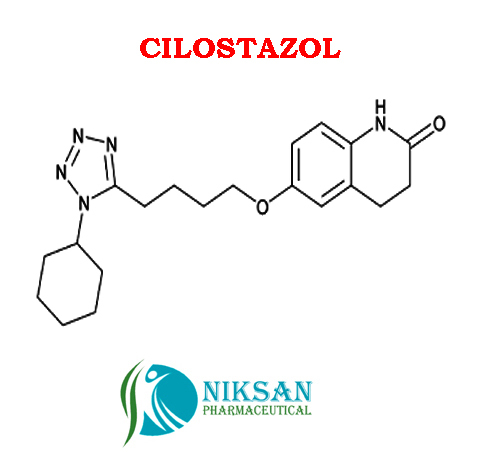
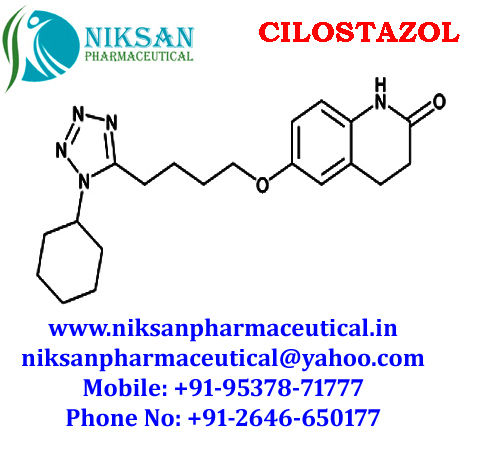
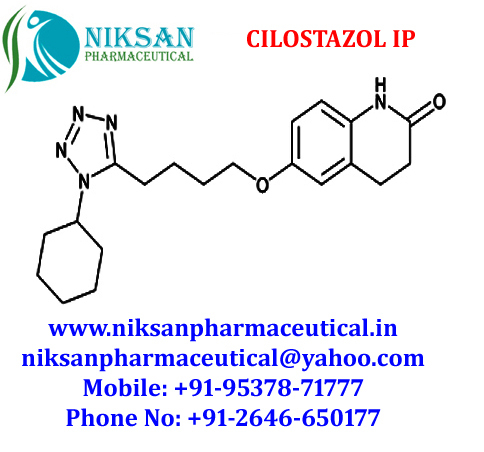
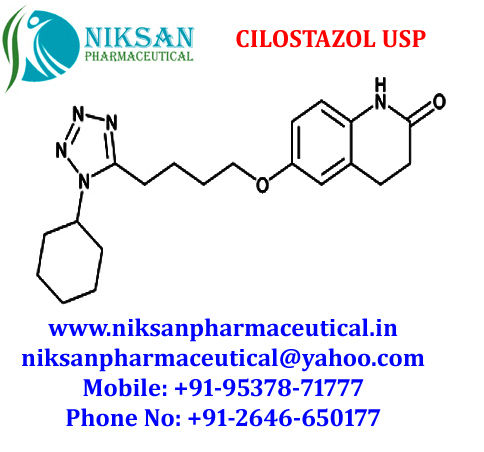
- Structural Formula C20H27N5O2
- EINECS No 73963-72-1
- Click to View more
CILOSTAZOL IP & CILOSTAZOL USP Price And Quantity
- 12500 INR/Kilograms
- 10 Kilograms
CILOSTAZOL IP & CILOSTAZOL USP Product Specifications
- 159.4-160.3
- White crystalline powder
- Room Temperature
- 10 MICRON
- 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone
- Cilostazol is a quinolinone-derivative medication used in the alleviation of the symptoms of intermittent claudication in individuals with peripheral vascular disease
- Cardiovascular Agents
- 99.8 %
- C20H27N5O2
- 0.5 %
- 664.7 C
- C20H27N5O2
- CILOSTAZOL IP & CILOSTAZOL USP
- 36 Months
- 0.5 %
- Powder
- 73963-72-1
- Medicine Grade
- 29420090
- 73963-72-1
- 369.46 Grams (g)
CILOSTAZOL IP & CILOSTAZOL USP Trade Information
- Janpt Navaseva
- 100 Kilograms Per Week
- 5 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Manipur, Himachal Pradesh, Nagaland, Uttarakhand, Daman and Diu, Dadra and Nagar Haveli, Lakshadweep, Jammu and Kashmir, Tripura, Andaman and Nicobar Islands, East India, Punjab, West India, Mizoram, West Bengal, Maharashtra, Sikkim, Jharkhand, Arunachal Pradesh, Telangana, Chandigarh, Meghalaya, Bihar, Tamil Nadu, South India, Rajasthan, Assam, Delhi, Karnataka, Goa, Pondicherry, Haryana, Uttar Pradesh, Gujarat, Madhya Pradesh, North India, Andhra Pradesh, Kerala, Central India, Odisha, Chhattisgarh, All India
- ISO, FDA, GMP, GLP
Product Description
NiksanPharmaceuticalprovides APIand finished formulations ofCilostazolin allover Indian States Like Kerala, Gujarat, Haryana, Rajasthan, Madhya Pradesh,Uttar Pradesh, Rajasthan, Karnataka, Meghalaya, Tamilnadu, Goa, Sikkim, Assam,Punjab, Delhi, Bihar, Jammu Kashmir Etc.
Niksanpharmaceuticalis alsolarge exporter of the API and finished pharmaceutical products ofCilostazolin manycountries for years. The countries where we exporting are Puerto Rico, Brazil,Honduras, Argentina, Nicaragua, Philippines, Colombia, Guatemala, Uruguay,Mexico, Chile, Dominican Republic, Panama, South Korea, United States, ElSalvador, Venezuela, Taiwan, Indonesia, Bolivia, Peru, Ecuador, Romania, Hong Kong,Thailand, United Arab Emirates, Iraq, Serbia, Spain, Bulgaria, Greece,Pakistan, Hungary, Portugal, Vietnam, Egypt, Iran, Poland, chia, Germany,Malaysia, Saudi Arabia, Canada, United Kingdom, Australia, Japan, Turkey, Italyand many more countries.
Cilostazolis belongs to drugs classcalledplatelet-aggregation inhibitors. It is works by improving bloodflow in legs. Cilostazol is anti-platelet type of drug and it also used asvasodilator.
Cilostazolstops blood cells to combine or sticktogether and inhibits blood clots. Cilostazol is used to increase the bloodflow because of that the Oxygen flow in body also increases. Cilostazol is onetype of vasodilators so it is also helps blood to flow smoothly in body.
SYNONYMS:Cilostazol,Cilostazole, Cilostazolum
IUPAC NAME:6-[4-(1-cyclohexyl-1H-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3, 4-tetra hydro quinolin-2-one
CAS NO:73963-72-1
FORMULA:C20H27N5O2
MOLECULRMASS:369.46 g/mol
STORAGE:Store it in cool and dry place, away from moistureand direct light. Does not store this medicine in bathroom or any humidly place.Keep medicine away from the reach of children and pets.
APPLICATIONS:Cilostazolis widely used as vasodilator.Cilostazolisalso use in the treatment of intermittent claudication.Cilostazolalsouse to treat cramp, ache, fatigue, classically it is used in calf muscle duringthe workout.
HOW TO USE:Follow all directions on your prescriptionlabel or as your doctor recommends you. Take Cilostazol two times per day on anempty stomach. Take medicine at least 45 minutes or 2 hours before meal.Cilostazoltakes time like 8-12 weeks to improve yoursymptoms. But if your symptoms do not improve after 3-4 weeks treatments tellyou doctor. Try to take medicine at a same time each day to improveeffectiveness.
HOWCILOSTAZOL WORKS:Cilostazolmetabolites are one type of phosphodiesteraseIII inhibitors (PDA III inhibitors).Cilostazolinhibits activity of phosphodiesterase and also quash CAMP degradationso by that CAMP increased in Blood vessels and platelets and by this activitythe platelet degradation inhibits and also blood clotting reduced by theactivity
CONTRAINDICATIONS:Do not take Cilostazol medicati on if youhave any heart problems like Congetive heart failure. Take your doctors adviceif you are pregnant or in lactation phase.Cilostazolcan harm nursing baby so do not take medication if you are breastfeedinga baby. Do not share your medication with other person. Take your doctors recommendationif any adverse reactions appear while taking the treatment ofCilostazol.
PHARMACOKINETICS:AbsorptionofCilostazolin gastrointestinal system is slow orincomplete.Cilostazolis absorbed after the oral administration. Ahigh fa meal can increase the absorption ofCilostazol. Thehalf-life of theCilostazolis between11-13 hours. TheCilostazolexcreted almost 74-76% by urination and theremaining medicine 23-27% eliminate by the faces like sweating or by skin.
SIDE EFFECTSOFCILOSTAZOL:The common side effects ofCilostazolare headache, upset bowel movements, runnynose, normal rash, dizziness. There are some major side effects are also causeby theCilostazollike difficulty in breathing, chest pain, swellingof (face, tongue, fingers, and throat). Take your doctors instructions at theminute if you have symptoms like Fever, chills, body aches, flu symptoms.Paining while urinating or bloody urine Shortness of breath, swelling of feetand fingers Chest pain, irregular heartbeats.
PRECAUTIONS:Do not takeCilostazolif you have heart problem.Cilostazolcan make your problem worst. For the safetytell your doctor if you have any problem related liver or kidney. Avoid grapefruitjuice or product. Do not takeCilostazolwith Batcaver it can increase the serum level.IfCilostazolistaken withAbciximab it can increase the severity of bleeding.
CDSCOAPPOVAL:CilostazolE.R. 100mg and 200 mg tablets are approved byCDSCO in India in30.10.2007.
FORMULATIONSAVAILABLE INMARKET:
Cilostazoletablets 50mg(E.R. release)
Cilostazoletablets 100mg(E.R. release)
Cilostazol+L-Carnitine100mg tablets
Note:Product protected by valid patents arenot offered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Pharmaceutical Excellence and GMP Compliance
Niksan Pharmaceutical delivers Cilostazol IP & USP formulations with strict adherence to GMP guidelines. We provide an unbroken chain of quality manufacturing, regular quality control checks, and well-maintained DMF documentation for seamless regulatory compliance. Our facilities guarantee top-notch standards for global pharmaceutical needs.
Versatile Dosage and Formulation Options
Our Cilostazol products are available as tablets (50 mg, 100 mg), capsules, and in raw powder form, ensuring flexibility and suitability for various applications. All products maintain high purity, optimal particle size, and are ideal for manufacturing cardiovascular and antihypertensive agents.
Proven Therapeutic Benefits
Cilostazol is widely recognized for its efficacy in managing intermittent claudication relating to peripheral vascular disease, supporting improved mobility and comfort for patients. Its precise structural formula ensures reliable pharmacological action and consistent results in clinical settings.
FAQs of CILOSTAZOL IP & CILOSTAZOL USP:
Q: How should Cilostazol IP & USP be stored to maintain stability?
A: Cilostazol should be stored in a well-closed container at room temperature to preserve its purity and effectiveness. Maintaining controlled storage conditions prevents moisture and contamination.Q: What dosage guidelines are recommended for Cilostazol tablets and capsules?
A: Cilostazol is typically available in 50 mg and 100 mg dosages for both tablets and capsules. Dosage must be determined by a medical professional, tailored to specific patient needs and health conditions.Q: What are the main ingredients and chemical specifics of Cilostazol?
A: The key ingredient is Cilostazol, with the chemical name 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone. The molecular formula is C20H27N5O2 and it has a purity of 99.8%.Q: When is Cilostazol typically recommended for use?
A: Cilostazol is primarily recommended for the management of intermittent claudication in individuals with peripheral vascular disease. It is classified as a cardiovascular agent with antihypertensive properties.Q: Where is Cilostazol manufactured and are regulatory documents available?
A: Niksan Pharmaceutical, based in India, manufactures and exports Cilostazol with GMP certification and provides a ready Drug Master File (DMF) for regulatory submissions and quality assurance.Q: What benefits does Cilostazol provide for patients with vascular conditions?
A: Cilostazol improves blood flow, reduces symptoms such as leg pain during walking, and enhances overall mobility, making it valuable for patients suffering from peripheral arterial disease.Q: What is the process for ordering Cilostazol in bulk from Niksan Pharmaceutical?
A: To order Cilostazol in bulk (500 kg unit), contact Niksan Pharmaceutical directly as a supplier, distributor, or exporter. All batches are subject to strict quality control and thorough documentation for export and regulatory needs.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese