AZ ILSARTAN
Product Details:
- Molecular Weight 568.53 g/mol Grams (g)
- EINECS No 808-058-6
- Solubility the solubility is approximately 0.1 mg/ml in ethanol, 3 mg/ml in DMSO, and 5 mg/ml in DMF.
- Taste Other
- Heavy Metal (%) NA
- Boiling point 118C
- Storage Room Temperature
- Click to View more
AZ ILSARTAN Price And Quantity
- 85000.00 INR/Kilograms
- 5 Kilograms
AZ ILSARTAN Product Specifications
- Room Temperature
- Other
- the solubility is approximately 0.1 mg/ml in ethanol, 3 mg/ml in DMSO, and 5 mg/ml in DMF.
- 118C
- NA
- Azilsartan represent the medicine class called angiotensin II receptor blockers (ARBs). It is widely use in treatment of hypertension (high blood pressure). Azilsartan lowers the blood pressure and helps to prevent heart attack and also use in prevention of kidney problems.
- Other
- 863031-21-4
- 568.53 g/mol Grams (g)
- 808-058-6
- Medicine Grade
- 192.4C to 195.7C
- White to Off-White Solid
- 29420090
- less than 20 micrometers for D90
- 3 Years
- Powder
- C30H24N4O8
- AZILSARTAN
- C30H24N4O8
- 99 %
- Other
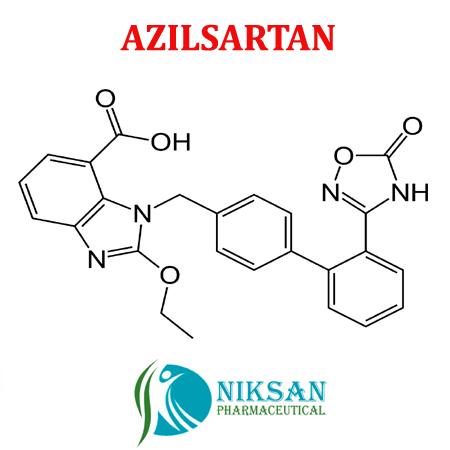
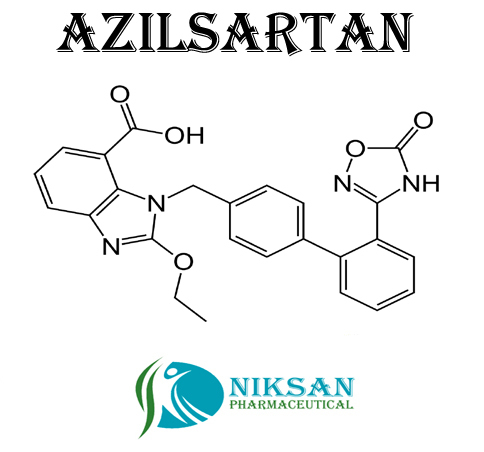
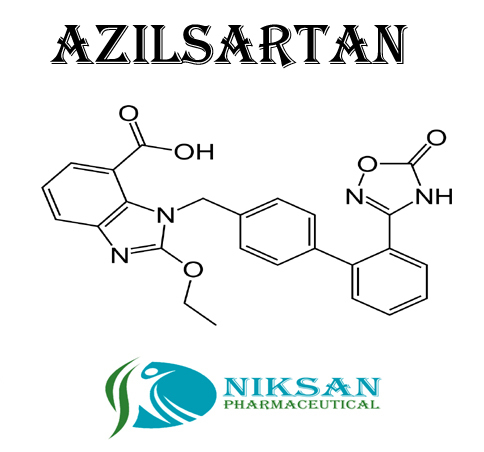
- 1-[[2-(2,5-Dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1-biphenyl]-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid
- between pH 1 and pH 7
- NA
AZ ILSARTAN Trade Information
- INDIA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINNR
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Himachal Pradesh, Andaman and Nicobar Islands, Dadra and Nagar Haveli, South India, Central India, North India, East India, West India, Andhra Pradesh, Assam, Arunachal Pradesh, Bihar, Chhattisgarh, Chandigarh, Goa, Haryana, Jammu and Kashmir, Daman and Diu, Delhi, Jharkhand, Karnataka, Kerala, Lakshadweep, Madhya Pradesh, Maharashtra, Mizoram, Meghalaya, Manipur, Nagaland, Odisha, Punjab, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Uttarakhand, West Bengal, Uttar Pradesh, Gujarat, All India
- FDCA, GMP, GLP AND ISO
Product Description
is the worlds leading pharmaceutical company in the manufacturing,supplying and exporting of AzilsartanTablets and API. Niksan Pharmaceutical provides wide range of API and Finishedpharmaceutical products of Azilsartanto the Niksan group companies and also to our customers in very affordableprice.
Niksan Pharmaceutical is manufacturer, supplier and exporter of the Azilsartan tablets in domestic and as well as worldwide.
Niksan Pharmaceutical are manufacturing and supplying Azilsartanin the all over the Indians states like Punjab, Tamilnadu, Gujarat, Delhi,Uttar Pradesh, Bihar, Rajasthan, Maharashtra, west Bengal, Assam, Odisha.
Niksan Pharmaceutical ismanufacturing and exporting Azilsartan fromlast many years in country like Portugal, Taiwan, United Arab Emirates, Mexico,Thailand, South Korea, China, United States, Canada, Germany, Vietnam, UnitedKingdom, Brazil, Egypt, Singapore, Jordan, Lebanon, Israel, Vietnam,Bangladesh, Paraguay, Argentina, Dominican Republic, Sudan, Hong Kong,Seychelles, Algeria, Iran, Bulgaria, Lithuania, Poland, Sri Lanka, Romania,Uruguay, Russia, Thailand, Afghanistan, Latvia, Lithuania, United ArabEmirates, Seychelles, Peru, Azerbaijan, Venezuela, Morocco, Cote D Ivories, StLucia, South Korea, Congo, Philippines, Colombia, Burkina Faso, Sudan, Guinea,Djibouti, Netherlands, Nigeria and Germany.
Azilsartan represent the medicine class called angiotensin II receptor blockers(ARBs). It is widely use in treatment of hypertension (high blood pressure).Azilsartan lowers the blood pressure and helps to prevent heart attack and alsouse in prevention of kidney problems.
SYNONYMS: Azilsartan,Azilsartan kamedoxomil, Azilsartan mdoxomil, Azilsartan Medoxomil, Azilsartnmedoxomilo, Azilsartanum medoxomilum
IUPAC NAME: (5-methyl-2-oxo-2H-1,3-dioxol-4-yl)methyl 2-ethoxy-1-({4-[2-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)phenyl]phenyl}methyl)-1H-1,3-benzodiazole-7-carboxylateCAS NO: 863031-21-4
FORMULA: C30H24N4O8
MOLECULAR MASS: 568.53 g/mol
STORAGE: Store the medicine in room temperature away from moisture and light.Keep the medicine in the original container. Keep medicine away from children and pets. Properlydiscard this product when it is expired or no longer needed.
APPLICATION: Azilsartan is widely used in the treatment of the hypertension, it isuse to lower the blood pressure in the body. It is used to prevent the heartstrokes. Azilsartan is also used in the treatment of the kidney disease.
HOW TO USE: The dosage is based on yourmedical condition and response to treatment. Take this medication by mouth asdirected by your doctor, usually once daily with or without food. Kindly dontforget to take the medicine.
CONTRAINDICATIONS: Avoid the Azilsartan with Aliskiren. When it is used with combinationwith the Aliskiren in the patient with diabetes there are several risk of theincrease of the adverse effects and also allergies. If any effect or allergyshown on the patient contact the prescriber or the nearby doctor.
SIDE EFFECTS: Normally the medicine is safe to use for the patient. But there are someadverse effect like diarrhoea, Dizziness, headache and also fatigue likesymptoms are shown in the patient some time in patient.
There is no any serious adverse reaction are shown in patient using theAzilsartan. But if you notice some serious allergic reactions like rash, itching, swelling of (mouth,tongue, eyes) or any problems comes in breathing kindly contact and take advice from yourdoctor.
ABSORPTION: The normal absorption time of Azilsartan is 1.5-3 hrs. Usually theAzilsartan is not affected by the other factors like food or any peak plasmaconcentrations. The time of volume of distribution of the Azilsartan in 16Litres. However the Azilsartan is a pro drug and the absolute bioavaibility ofthe Azilsartan in 60%.
TOXICITY: There is no any major toxic effects are known of Azilsartan however sometime headache, problem in breathing are shown take the advice of your doctor.
CDSCO APPROVAL: Azilsartan formulation is approved by CDSCO in India in 16.02.2016Azilsartan 40mg tablet and Azilsartan 80mg tablet.
FORMULATIONS IN MARKET:
Azilsartan 40mgtablet
Azilsartan 80mgtablet
Azilsartan Medoxomilsalt tablet
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
SAHAR AIR CARGO
FDCA, GMP, GLP, ISO
DOUBLE LDPE LINERS IN HDPE CARBOYS

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese