PR EDNISOLONE
Product Details:
- EINECS No 200-021-7
- Smell Other
- Molecular Formula C21H28O5
- Boiling point 579.8 C at 760 mmHg
- Ph Level 2.6-3.6
- HS Code 30043912
- Heavy Metal (%) NA
- Click to View more
PR EDNISOLONE Price And Quantity
- 1 Kilograms
- 90000.00 INR/Kilograms
PR EDNISOLONE Product Specifications
- Other
- Pharmaceutical Intermediates
- 200-021-7
- White to Off-White Solid
- 2.6-3.6
- C21H28O5
- 579.8 C at 760 mmHg
- Powder
- NA
- Other
- Keep away from moisture
- 99 %
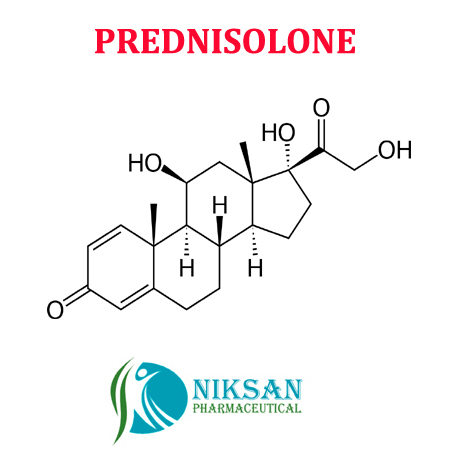
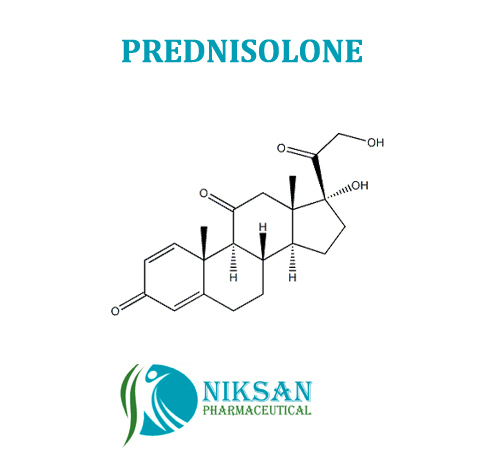
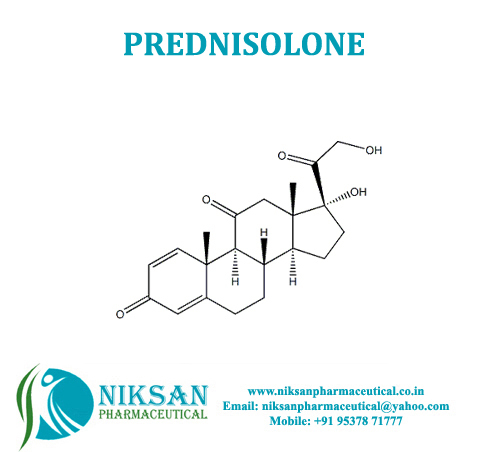
- 11,17,21-trihydroxypregna-1,4-diene-3,20-dione
- 30043912
- 360.44 g/mol Grams (g)
- very slightly soluble in water
- PREDNISOLONE
- Prednisolone is steroid medicine which is used to treatment of allergies, inflammatory conditions, autoimmune disorders, and cancers.
- 235 C (455 F)
- not lose more than 1.0%
- 50-24-8
- 3 Years
- nanometers to micrometers
- C21H28O5
- Medicine Grade
PR EDNISOLONE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan pharmaceutical is one of the leading manufacturer, supplier, distributor and exporter of Prednisolone API and finished formulation as all dosage forms. Our product Prednisolone is widely used and appreciated by our group companies and also our customers and users all around the nations. We offer the Prednisolone in very affordable price.
Niksan Pharmaceutical is the suppliers, manufacturer, exporter and trader in the domestic level as well as the international market.
Niksan Pharmaceutical provides Prednisolone API in all over Indian states like Uttara hand, Pondicherry, Kerala, AndhraPrades, Punjab, Himachal Pradesh, Chhattisgarh, Jammu & Kashmir, Haryana, Telangana, Goa, TamilNadu, Jharkhand, Karnataka, Rajasthan, Chandigarh, Gujarat, Maharashtra, UttarPradesh, Odisha, Delhi, West Bengal, Assam, Manipur, Bihar, Madhya Pradesh and many other state.
Niksan Pharmaceutical also exports Prednisolone API in world other countries like Zimbabwe, Trinidad & Tobago, Zambia, Ghana, Kenya, Singapore, Mauritius, Australia, Malaysia, Tanzania, Uganda, Nigeria, United Kingdom, Ireland, Puerto Rico, Iraq, Sri Lanka, France, Nepal, Sudan, Jordan, Thailand, Qatar, Oman, United Arab Emirates, Hong Kong, Taiwan, United States, Kuwait, Bangladesh, Saudi Arabia, Belgium, Pakistan, Morocco, Philippine, Algeria, Canada, New Zealand, South Africa, South Korea, Egypt, Norway, Indonesia, Iran, Switzerland, Vietnam, Greece, Denmark, Israel and many other countries.
Prednisolone is steroid medicine which is used to treatment of allergies, inflammatory conditions, autoimmune disorders, and cancers.
SYNONYMS: Delta-Dehydrocortisol, Delta-Dehydrohydrocortisone, Delta-Hydrocortisone, Delta (1)-Dehydrocortisol, Delta (1)-Hydrocortisone, Hydroretrocortine, Metacortandralone, Prdl, Prednisolona, Prednisolone, Prednisolonum
IUPAC NAME: (1R,3aS,3bS,9aR,9bS,10S,11aS)-1,10-dihydroxy-1-(2-hydroxyacetyl)-9a,11a-dimethyl-1H,2H,3H,3aH,3bH,4H,5H,7H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-7-one
CAS NO: 50-24-8
FORMULA: C21H28O5
MOLECULAR MASS: 360.44 g/mol
APPLICATIONS OF Prednisolone: Prednisolone is used to treatment of immune system disorders, skin and eye conditions, arthritis, blood problems, breathing problem and cancer. It is used to treatment of some allergic reaction.
HOW TO USE: Take single dose in one day. Prednisolone is available as form of solutions and tablet so you can take by mouth with or without food.
HOW PREDNISOLONE WORKS: Prednisolone work by lowering the activity of the immune system.
CONTRAINDI CATIONS: If you have any untreated tuberculosis, inactive tuberculosis, herpes simplex infection of the eye, a herpes simplex infection and an infection due to a fungus kindly avoid Prednisolone. If you have low amount of potassium or magnesium in your blood or body kindly avoid Prednisolone.
PHARMA COKINETICS OF PREDNISOLONE: Absorption is approximately 70%after oral administration. Half-life of Prednisolone is 2.1 to 3.5 hours. Peak plasma concentration of Prednisolone is approximately 113-1343mg/ml. 98%Prednisolone eliminate via urine.
SIDE EFFECTS OF Prednisolone: Common side effect of Prednisolone is weight gain, Indigestion, sleep problems, Restlessness and sweating a lot.
PRECAUTIONS: Before using this medication, tell your doctor or pharmacist your medical history. This drug may make you dizzy so avoid alcohol and do not drive and do not handle machinery. In the rare case you may infected by Prednisolone. This medication may effect on children's height.
CDSCO APPROVAL: Levofloxacin(15mg) + Prednisolone Acetate 10mg per ml eye drops are approved by CDSCO in India in 11.07.2007
Clotrimazole (10mg) + Methylprednisolone acoelomate 1mg/gm of cream is approved by CDSCO in India in 12.07.2007
Docetaxal injection is approved by CDSCO in India in 09.03.2005
MoxifloxacinHCl (5mg) + Prednisolone Acetate (10mg) per ml. (eye drops) are approved by CDSCO in India in 16.10.2007
Prednisolone stearoyl glycolate tablet is approved by CDSCO in India in November-1970
Mupirocin(2%) + Methyl prednisolone Aceponate (0.1%) Cream is approved by CDSCO in India in31.10.2007
Methylprednisolone aceponate-1mg + salicylic acid-50mg tablet is approved by CDSCO in India in 19.11.2007
Methyl prednisolone aceponate-1mg + Mupirocin-20mg cream is approved by CDSCO in India in 21.11.2007
Prednisolone Sodium Phosphate Orally Disintegrating Tablet is approved by CDSCO in India in26.11.2009
Prednisolone tablet is approved by CDSCO in India in December-1971
Methyl prednisolone tablet is approved by CDSCO in India in March-1978
Methyl prednisolone acetate tablet is approved by CDSCO in India in November-1962
Methyl prednisolone Tablet 8mg is approved by CDSCO in India in 01.09.2009
Prednisolone+ Ofloxacin tablet is approved by CDSCO in India in 2003
Methylprednisolone Acrponate topical solution 0.1%is approved by CDSCO in India in 09.04.2007
Gatifloxacin( 0.3%) + Prednisolone Acetate (1%) eye drops are approved by CDSCO in India in 02.06.2006
Prednisolone stearoyl glycolate tablet is approved by CDSCO in India in February-1971
Methylprednisolone Acoelomate Cream (0.1%) is approved by CDSCO in India in 15.06.2006
Lincomycin +Neomycin + Methyl prednisolone is approved by CDSCO in India inMarch-1989
FORMULATIONS AVAILABLE IN MARKET:
Prednisolone 10 MG tablets
Prednisolone 5 MG tablets
Prednisolone 1 %W/V solutions
Prednisolone 30 MG tablets
Prednisolone 20 MG tablets
Prednisolone 40 MG tablets
Prednisolone 1 MG tablets
Prednisolone 2 MG tablets
Ofloxacin 3MG+Prednisolone 10 MG /ML solutions
Prednisolone 5 MG /5ML solutions
Prednisolone 15 MG /5MLsolutions
Prednisolone 2.5 MG tablet
Moxifloxacin 0.5 %W/V+Prednisolone 1 %W/V solutions
Gatifloxacin 0.3 %W/V+Prednisolone 1 %W/V solutions
Chloramphenicol 0.5 %W/V+Prednisolone 0.1 %W/V solutions
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese