- Home Page
- Company Profile
-
Our Products
- Active Pharmaceuticals Ingredients (API)
- CILNIDIPINE
- LITHIUM CARBONATE
- TRAZODONE
- CLOBETASOL PROPIONATE
- FEBUXOSTAT
- TORSEMIDE
- QUETIAPINE
- MINOXIDIL .
- NORETHINDRONE
- METOCLOPRAMIDE
- MELATONIN .
- PIRO XICAM
- ORN IDAZOLE
- LOPERAMIDE
- FENBENDAZOLE
- FLUCONAZOLE
- LACOSAMIDE
- LEVOSULPIRIDE
- FEXOFENADINE
- LAFUTIDINE .
- DIACEREIN
- CELECOXIB
- NORETHISTERONE ACETATE
- BIPERIDEN
- SUMATRIPTAN SUCCINATE
- LEFLUNOMIDE
- BENFOTIAMINE
- GLIMEPIRIDE
- GRANISETRON HCL
- DONEPEZIL .
- SOLIFENACIN SUCCINATE
- FINASTERIDE
- FLUOCINOLONE ACETONIDE
- ETORICOXIB IP/BP/USP
- MEMANTINE
- ARTEMETHER
- AMISULPRIDE
- SILODOSIN
- PRIMIDONE
- PRAZIQUANTEL
- IGURATIMOD
- MOXIFLOXACIN HCL
- BISOPROLOL
- LERCANIDIPINE
- LUMEFANTRINE
- DOXOFYLLINE .
- ROSUVASTATIN
- ONDANSETRON
- ERYTHROMYCIN
- ADAPALENE
- DOMPERIDONE
- KETOPROFEN
- ITOPRIDE
- CLARITHROMYCIN .
- LINAGLIPTIN
- CLOSANTEL SODIUM
- AZILSARTAN
- NICORANDIL
- GLICLAZIDE
- CALCIUM LYSINATE
- CILOSTAZOL
- SITAGLIPTIN
- LORNOXICAM
- CITICOLINE
- RIVAROXABAN
- FLUOROMETHOLONE
- LULICONAZOLE
- METOPROLOL
- ALLOPURINOL
- AMBROXOL
- DEFLAZACORT
- BENIDIPINE
- BILASTINE
- ATORVASTATIN
- CILOSTAZOL IP & CILOSTAZOL USP
- FENOFIBRATE
- DESLORATADINE
- BRIVARACETAM
- EMPAGLIFLOZIN
- DROTAVERINE
- AMANTADINE
- PREDNISOLONE
- IVERMECTIN
- EDARAVONE
- OLMESARTAN
- ESOMEPRAZOLE
- MONTELUKAST
- TAMSULOSIN
- RASAGILINE
- VOGLIBOSE
- URSODEOXYCHOLIC ACID
- TENELIGLIPTIN
- SEVELAMER CARBONATE
- TERBINAFINE
- LETRAZOLE
- PROGESTERONE
- PREGABALIN
- METHYL PREDNISOLONE
- NA TEGLINIDE
- NI FEDIPINE
- GABAP ENTINE
- NE BIVOLOL
- LA BETALOL
- BECLOMETHASONE DIPROPIONATE
- FE BANTEL
- LE VETIRACETAM
- ALPHA LIPOIC ACID
- DABIGATRAN
- NORETHINDRONE ACETATE
- Dapoxetine
- Nandrolone Phenylpropionate
- Orlistat
- Tadalafil
- BO SENTAN
- PERMETHOL SODIUM
- BACLOFEN IP
- DULOXETINE
- Pharma Intermediates
- BENIDIPINE INTERMEDIATE
- TORSEMIDE INTERMEDIATES
- 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate
- 2(2- ETHOXY PHENOXY ) ETHYL BROMIDE
- LULICONAZOL INTERMEDIATES
- 3-amino Crotonicacid Cinnamyl Ester
- FENBENDAZOLE INTERMEDIATES
- (2R)-rel-6-Fluoro-3,4-dihydro-2-(2R)-2-oxiranyl-2H-1-benzopyran
- Febantel Intermediates
- 4-(3'-Methylphenyl)amino-3-pyridinesulfonamide
- 4-Chloro-3-Pyridine Sulfonamide
- 2-Methoxyethyl-(3-Nitrobenzyledene) Acetoacetate
- (R)-(-)-3-Carbamoymethyl-5-methylhexanoic acid
- SILODOSIN INTERMEDIATES
- DAPAGLIFLOZIN
- 2, 6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic Acid
- 1-(3-Methyl-1-phenyl-5-pyrazolyl) piperazine
- 5-Chloro-2-Nitro Aniline
- 3-Nitrobenzaldehyde
- IMIDAZOL-1-YL-ACETONITRILE
- SILODOSIN INTERMEDIATE
- LERCANIDIPINE INTERMEDIATE
- 6-Hydroxy-2(1H)-3,4-dihydroquinolinone
- MANIDIPINE INTERMEDIATE
- Pranidipine Intermediate
- BARNIDIPINE INTERMEDIATES
- Nicardipine Intermediate
- (S)-2-Chloro-1-(2,4-dichlorophenyl)ethanol
- MEPIRODIPINE INTERMEDIATE
- 5-(4-Chlorobutyl)-1-cyclohexanyl tetrazole
- (2R)-rel-6-Fluoro-3,4-dihydro-2-(2S)-2-oxiranyl-2H-1-benzopyran
- 2-Nitro 5-Phenyl Mercapto Aniline
- 1-Benzyl-3-hydroxypiperidine
- TENELIGLIPTIN INTERMEDIATES
- (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester
- PREGABALIN INTERMEDIATE
- 3-Carbamoymethyl-5-methylhexanoic acid
- 5-2r-2-aminopropyl-1-3-benzoyloxy-propyl-2-3-dihydro-1h-indole-7-carbonitrile-2r-3r-2-3-dihydroxybutanedioate
- TAMSULOSIN INTERMEDIATES
- NEBIVOLOL INTERMEDIATES
- 1-(3-Methyl-1-phenyl-5-pyrazolyl)piperazine acetate salt
- 5-((2R)-2-AMINOPROPYL)-2-METHOXYBENZENE SULFONAMIDE
- CILOSTAZOL INTERMEDIATES
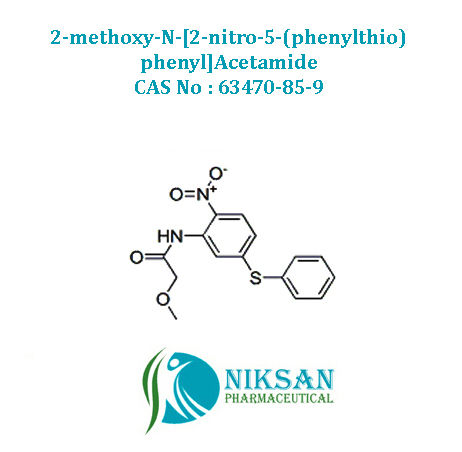
- 2-methoxy-N-[2-nitro-5-(phenylthio)phenyl]Acetamid
- Cilnidipine Intermediates
- Dapoxetine IP
- 1,3-Bis(Methoxycarbonyl)-2-Methyl-2-Thiopseudouera
- Pharmaceutical Formulation
- Tretinoin Cream
- Glipezide 2.5 Mg Tablets
- Salbutamol 4 Mg Tablets
- Febendazole 3GM BOLUS
- Lumefantrine 120Mg Artemether 20Mg Tablets
- Candesartan Cilexetil 16 Mg Tablets
- Omeprazole 20 Mg Capsules
- Gabapentin 800 Mg Tablets
- Enzymes Drops
- Oral Rehydration Salts
- Metoprolol Tartrate 25Mg Sustain Release Tablets
- Hydrocortisone Acetate Fusidic Acid Cream
- POVIDONE IODINE USP OINTMENT
- Telmisartan 20 Mg Tablets
- Nimodipine 30 Mg Tablets
- Amoxycillin Trihydrate Dicloxacillin Capsules
- Beclomethasone Propionate Clotrimazole Neomycin Cream
- Praziquantel Pyrantel Pamoate And Fenbendazole SUSPENSION
- Benzoyl Peroxide Cream
- Atorvastatin 20 Mg Tablets
- Diclofenac Sodium 75 Mg Tablets
- Vitamin D3 Drops
- Piroxicam Gel Bp
- SUNSCREEN CREAM 50 SPF
- Silodosin 8 Mg Capsules
- Fluconazole 150 Mg Tinidazole 1000 Mg Tablets
- Montelukast Sodium 10 Mg Bambuterol 10 Mg Tablets
- Omeprazole 20 Mg Domperidone 10 Mg Capsules
- Fusidic Acid Beclomethsone Dipropionate Cream
- Dihydralazine 25Mg Tablets
- Chloroquine 250Mg Tablets
- Ketoconazole 200 Mg Tablets
- Beta Carotene Vitamin A Vitamin E Vitamin C Tablets
- Bisoprolol 2.5 Mg Amlodipine 5 Mg Tablets
- Fexofinadine Syrup
- Levosulpride 50 mg tablets
- Levofloxacin 250 Mg Tablets
- Fluconazole 50 Mg Capsules
- Metformin Hcl 500 Mg Sustain Release Tablets
- Fluconazole 0.5 % Cream
- Carbonyl Iron Folic Acid Zinc Sulphate Vitamin B12 Tablets
- Pepsin Diastase Tablets
- Itraconazole 100 Mg Capsules
- Diloxanide 500Mg Tablets
- Ofloxacin 100 Mg Tablets
- Ofloxacin 200 Mg Tinidazole 600 Mg Tablets
- Voglibose 0.3 mg tablets
- Lisinopril 5 Mg Amlodipine 5 Mg Tablets
- Isosorbide Mononitrate 5 Mg Tablets
- Clotrimazole Ip 1% Soap
- Glimepride 1 Mg Tablets
- Losartan Potassium Hydrochlorothiazide Tablets
- Praziquantel Pyrantel Pamoate And Fenbendazole Bolus
- Ferric Ammonium Citrate Vitamin B-12 Folic Acid Syrup
- Amlodipine 5 Mg Losartan 50 Mg Tablets
- Ambroxol Guaiphenesin Levosalbutamol Oral Drops
- Paracetamol 500 Mg Domperidone 20 Mg Tablets
- Choline Salicylate Lignocaine Hcl Benzalkonium Gel
- Diclofenac Sodium 1 % W/w Gel
- Ofloxacin Metronidazole Simethicone Suspension
- Pantoprazole 40mg Levosulpiride 75mg (Sr) Capsules
- Glucosamine Diacerein Capsicum Oleoresin Menthol Gel
- Pregabalin Capsules 50 Mg
- Ketoconazole Idhq Tolnaftate Gentamicin Clobetasol Cream
- Pantoprazole 20 Mg Tablets
- Artesunate 100 Mg Mefloquine 220 Mg Tablets
- Mupirocin 2.0 % W/W Ointment
- Glimepride 2 Mg Tablets
- Clarithromycin 125Mg Dry Syrup
- Ketoconazole Soap 2% W/W
- Chlorthalidone 12.5 Mg Tablets
- Metronidazole Chewable 250mg Tablets
- Pantoprazole 40mg Itopride 150mg (Sr Pellets) Capsules
- Clindamycin Benzoyl Peroxide Gel
- Dicyclomine 20 Mg Paracetamol 500 Mg Tablets
- Amlodipine 2.5Mg Tablets
- Famotidine 20 Mg Tablets
- Cefpodoxime Dry Syrup
- Famotidine 40 Mg Tablets
- Paracetamol 100 Mg Dispersible Tablets
- Serratiopeptidase 10mg Diclofenac 50mg Tablets
- Pyrazinamide Tablets 400 Mg
- Ibuprofen 400 Mg Tablets
- Dicyclomine 10 Mg Diclofenac Potassium 50 Mg Tablets
- Atenolol 25 Mg Tablets
- Rosuvastatin 5mg Tablets
- Torsemide 20 Mg Tablets
- Cefixime Ofloxacin Dry Syrup
- Vitamin B1 B2 B6 B12 C Biotin Calcium Capsules
- Ambroxol Levocetrizine Syrup
- Gabapentin 300 Mg Mecobalamin 500 Mcg Tablets
- Montelukast Levocetrizine Syrup
- Artesunate 100Mg Tablets
- Drotaverine 40Mg Tablets
- Mefloquine 250 Mg Tablets
- Nicorandil 10 Mg Tablets
- SILVERSULPHADIAZINE AND CHLORHEXIDINE CREAM
- Folic Acid Zinc Sulphate Ferrous Sulphate Sr Capsules
- FEBUXOSTATE Tablets 40 Mg
- Benzydamine Hcl Mouth Wash
- Dicyclomine Hydrochloride Pediatric Drops
- Acarbose 100 Mg Tablets
- Amodiaquine 200 Mg Tablets
- Cyproheptadine Tricholine Citrate Sorbitol Syrup
- Diclofenac Linseed Oil Benzyl Alcohol Gel
- Albendazole Chewable 200 Mg Tablets
- Ibuprofen Paracetamol Tablets
- Acetazolamide 250 Mg Tablets
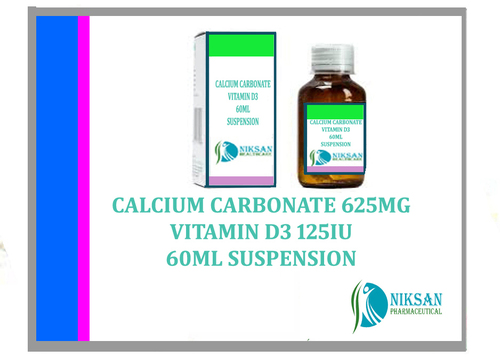
- Calcium Carbonate Vitamin D3 125Iu Suspension
- Bacitracin Zinc Neomycin Polymixin B Sulphate Ointment
- Aceclofenac 200 Mg Tablets
- Artesunate Sulphadoxin Pyrimethamine Tablets
- SUCRALFATE LIGNOCAINE CREAM
- Atenolol Lostartan Tablets
- Salicylic Acid Face Wash
- Tinidazole 300 Mg Tablets
- Paracetamol Phenylepherine Cetrizine Zinc Gluconate Syrup
- Artesunate 50Mg Tablets
- Itraconazole 200 Mg Capsules
- Cefixime Dry Syrup
- Flunarizine Domperidone Paracetamol Tablets
- Domperidone 10 Mg Tablets
- Ambroxol Salbutamol Syrup
- Glipezide 5 Mg Metformin 500 Mg Tablets
- Flunarizine 5 Mg Tablets
- Atenolol 50 Mg Tablets
- Moxifloxacin Hcl 400 Mg Tablets
- Secnidazole Film Coated 500mg Tablets
- Ethionamide 250 Mg Tablets
- Clindamycin Phosphate Gel
- Aceclofenac Tablets 100 Mg
- Isosorbide Dinitrate 40 Mg Tablets
- Diltiazem 90Mg Sustain Release Tablets
- Carbonyl Iron Folic Acid Cyanocobalamine Zinc Syrup
- Paracetamol Suspension Bp
- MICONAZOLE CREAM
- Ketoconazole 2 % W/w Cream
- Mefenamic Acid 500 Mg Tablets
- Lansoprazole 30 Mg Tablets
- Loperamide Hcl 2 Mg Tablets
- Nicorandil 5 Mg Tablets
- Clopidogrel Bisulphate 75 Mg Tablets
- Amoxycillin Potasium Clavunate Syrup
- Rabeprazole Sodium (Ec) Levosulpride (Sr) Capsules
- Artemether 180 Mg Lumefantrine 1080 Mg Tablets
- Alpha Amylase Papain 30 Ml Syrup
- Levosulbutamol Ambroxol & Guaphensin Syrup
- Metoprolol Tartrate 100 Mg Sustain Release Tablets
- Lansoprazole 15 Mg Tablets
- Megaldreate Simethicone Domperidone Suspension
- Repaglinide 2 Mg Tablets
- Norfloxacin 400 Mg Tinidazole 600 Mg Tablets
- Aceclofenac 100 Mg Paracetamol 500 Mg Tablets
- Amiodarone 200 Mg Tablets
- Duloxetine Hcl 20Mg Tablets
- Benzoyl Peroxide Clindamycin Phosphate Soap
- Valsartan 40mg Tablets
- Dextromethorphan Cpm Guaiphenesin & Ammonium Syrup
- Ofloxacin Ornidazole 30 Ml Suspension
- Diacerein Linseed Oil Methylsalicylate Menthol Ointment
- Diclofenac Linseed Oil Thiocolchicoside Menthol Gel
- Diltiazem 30Mg Tablets
- Paracetamol 500 Mg Caffeine 65 Mg Tablets
- Clobetasol Propionate Miconazole Neomycin Zinc Cream
- Pantoprazole 40 Mg Tablets
- Metformin Hcl 1000 Mg Tablets
- Mefenamic Acid 500 Mg Paracetamol 450 Mg Tablets
- Carvedilol 12.5 Mg Tablets
- Calcium 250 Mg Vitamin D3 125 Iu Tablets
- Clobetasole Miconazole Gentamycin Zinc Oxide Lotion
- B Complex Multivitamin With L Lysine Syrup
- Amlodipine 5 Mg Tablets
- CILOSTAZOLE 100 MG Tablets
- Iron Folic Acid Vitamin B12 Capsules
- cilecoxib 100 mg Capsules
- Amlodipine 10 Mg Tablets
- Artemether Capsules 40 Mg
- Torsemide 10mg Tablets
- Flavoxate Hcl 200Mg Tablets
- Cefixime Potasium Clavunate Dry Syrup
- Levofloxacin 500 Mg Tablets
- Ondansetron 4 Mg Paracetamol 500 Mg Tablets
- Theophylline 400 Mg Controlled Release Tablets
- Roxithromycin 50 Mg Tablets
- Norfloxacin 100 Mg Tablets
- TELMISARTAN 40 MG RAMIPRIL 2.5 MG TABLETS
- Aceclofenac Paracetamol Suspension
- LULICONAZOLE 1 % W/W CREAM
- Theophylline 200 Mg Salbutamol 4 Mg Tablets
- Dextromethorphan Chlorpheniramine Phenyephrine Syrup
- Levocetrizine Syrup
- Clindamycin Adapalene Gel
- Adapalene Gel
- Azithromycin 250 Mg Capsules
- Diclofenac Aloe Vera Methyl Salicylate Menthol Gel
- Ciprofloxacin Cream
- Clotrimazole Beclomethasone Neomycin Cream
- Bromhexine Guaiphenesin Terbutaline Sulphate 100 Ml Syrup
- CLOTRIMAZOLE BECLOMETHOSONE NEOMYCIN CHLOROCRESOL CREAM
- Haemitinic Capsules
- Metoclopramide 5Mg Paracetamol 500Mg Tablets
- Cetirizine 5 Mg Tablets
- Labetalol 100 Mg Tablets
- Amoxycillin 250 Mg Capsules
- Levofloxacin 500 Mg Ornidazole 1Gm Tablets
- Metoprolol Tartrate 25 Mg Amlodipine 5Mg Tablets
- Terazosin 2 Mg Tablets
- Frusemide 40 Mg Tablets
- Montelukast Sodium 5 Mg Tablets
- Doxofylline 400Mg Tablets
- TERBINAFINE HYDROCHLORIDE CREAM
- Esomeprazole Clarithromycin Amoxycillin Tablets
- Calcium Carbonate Magnesium Zinc Vitamin D3 Suspension
- Finasteride 5mg Tablets
- Hydrochlorothiazide 25Mg Tablets
- Cefixime Azithromycin Dry Syrup
- Lactic Acid Bacillus 60 Million Spores Capsule
- Amisulpride 50 mg tablets
- Enalapril Maleate 10 Mg Hydorchlorthiazide 15 Mg Tablets
- FEBENTEL 75mg Tablets
- Cholecalciferol Granules 60,000 I.U
- Rosuvastatin 10 Mg Tablets
- Eberconazole 1 % W/w Cream
- Mecobalamin 1500 Mcg Capsule
- Fusidic Acid Cream
- Tetracycline 50 Mg Dispersible Tablets
- Amlodipine 5 Mg Lisinopril 5 Mg Tablets
- Cinnarizine 75 Mg Tablets
- Ethambutol 400 Mg Tablets
- Ketoconazole 2 % V/V Shampoo
- Cinnarizine 25 Mg Tablets
- Tetracycline 500 Mg Tablets
- Candesartan Cilexetil 8 Mg Tablets
- Frusemide 40 Mg Amiloride 5 Mg Tablets
- Glycerin 15 % W/w Cream
- Sucralfate Tinidazole Povidone Iodine Ointment
- Benzoyl Peroxide 2.5 % W/w Gel
- Glibenclamide 5 Mg Tablets
- Sucralfate Oxcetacain 60Ml Suspension
- Itraconazole Dusting Powder
- Losartan Potassium 25 Mg Tablets
- Amlodipine 5Mg Atenolol 25Mg Tablets
- Azithromycin 200Mg Dry Syrup
- OFLOXACIN ORNIDAZOLE TERBINAFINE CLOBETASOL CREAM
- Rabeprazole Sodium 20 Mg Tablets
- Acyclovir 200 Mg Tablets
- Norfloxacin 400 Mg Metronidazole 500 Mg Tablets
- Phenylephrine Chlorpheniramine Maleate Paracetamol Drops
- Chlorhexidine Gluconate Salicylic Acid Soap
- Azilsartan 40mg Tablets
- Pantoprazole Domperidone Prologed Releases Capsules
- Theophylline 200 Mg Tablets
- Paracetamol Cetrizine Hcl Phenylephrine Hcl Suspension
- Metformin Hcl 500 Mg Tablets
- Lycopene Multi Vitamins Multi Minerals 200 Ml Syrup
- Azithromycin 100 Mg Tablets
- Tetracycline 250 Mg Capsules
- Minoxidil Solution
- Vitamin E Acetate Cream
- SODIUM FUSIDATE CREAM
- Bosentan 125 mg Tablets
- Diclofenac Sodium 50 Mg Tablets
- Amlodipine Atorvastatin Tablets
- Levocetrizine Phenylepherine Syrup
- Lactobacilli Thiamine Riboflavin Pyridoxine Niacin Tablets
- Dha-200mg Folic Acid 1.5mg Capsule
- Cinnarizine 15Mg + Domperidone 15 Mg Tablets
- Losartan Potassium 50 Mg Tablets
- Isosorbide Mononitrate 10 Mg Tablets
- Metoclopramide Hcl 10 Mg Tablets
- Frusemide 40 Mg Spirionlactone 50 Mg Tablets
- Dexrabeprazole Domperidone (Sr Pellets) Capsules
- Praziquantel Pyrantel Pamoate And Fenbendazole SYRUP
- Vitamin- C 50mg Tablets
- Multivitamin Multimineral 200 Ml Syrup
- Paracetamol 500 Mg Tablets
- Lansoprazole 30 Mg Domperidone 10 Mg Tablets
- Mefenamic Acid Paracetacetamol Suspension
- Simvastatin 10 Mg Tablets
- Nebivolol 5 Mg Tablets
- Losartan Potassium 50 Mg Amlodipine 5 Mg Tablets
- Clindamycin Nicotinamide Gel
- Aceclofenac Paracetamol Rabeprazole Tablets
- SERTACONAZOLE NITRATE BECLOMETHASONE DIPROPIONATE CREAM
- Azathioprine 50 Mg Tablets
- Salbutamol 2 Mg Tablets
- Telmisartan 40 Mg Hydrochlorothiazide 12.5 Mg Tablets
- Cilnidipine 10 Mg Tablets
- Ciprofloxacin 100 Mg Tablets
- Silodosin 4Mg Capsules
- Haemitinic Syrup
- Isosorbide Dinitrate 10 Mg Tablets
- Amiodarone 100 Mg Tablets
- Carvedilol 6.25 Mg Tablets
- Pregabalin Capsules 75 Mg
- Esomprazole 40mg Domperidone 20mg (Sr Pellets) Capsules
- Ofloxacin 400 Mg Ornidazole 600 Mg Tablets
- Paracetamol Phenylepherine Cpm Sodium Citrate Syrup
- Gabapentin Lignocaine Methyl & Propyl Paraben Ointment
- Bisoprolol 2.5 Mg Tablets
- Lactic Acid Vaginal Wash
- Glibenclamide 5 Mg Metformin 500 Mg Tablets
- Artesunate 100 Mg Amodiaquine 270 Mg Tablets
- Nimesulide 100 Mg Paracetamol 500 Mg Tablets
- Atenolol 50Mg Nifedipine 20Mg Tablets
- Hydroquinone Tretinoin Momentasone Cream
- Nebivolol 2.5 Mg Tablets
- Diclofenac 50Mg Paracetamol 500Mg Tablets
- Simvastatin 5 Mg Tablets
- Carvedilol 3.125 Mg Tablets
- Cyproheptadine 1.5 Mg Tablets
- Folic Acid 1 Mg Tablets
- Fluconazole & Chlorhexadine Hcl Powder
- Linezolid Dry Syrup
- Voglibose 0.3 mg Metformin 500 mg tablets
- OFLOXACIN CLOBETASOL ORNIDAZOLE ITRACONAZOLE CREAM
- Ondansetrone 4 Mg Tablets
- Hydrochlorothiazide 12.5Mg Tablets
- FEBENTEL 150 Mg tablets
- TACROLIMUS OINTMENT
- Aripiprazole Tablet 5 Mg
- Isosorbide Dinitrate 20 Mg Tablets
- Chlorthalidone 100 Mg Tablets
- Etoricoxib 90 Mg Tablets
- Enzymes Syrup
- Allopurinol 100 Mg Tablets
- Amoxycillin Dry Syrup
- Diltiazem 240 Mg Sustain Release Tablets
- Dexamethasone 1 Mg Tablets
- Clobetasol Propionate Salicylic Acid Ointment
- Piracetam Tablets 400 Mg
- Sparfloxacin 100 Mg Tablets
- Etoricoxib Thiocolchicoside tablets
- Telmisartan 40 Mg Tablets
- Febuxostate Eye Drop
- Praziquantel Pyrantel Pamoate And Febentel Tablet
- Ethamsylate 250 Mg Tablets
- Olmesaratan 20mg Tablets
- Clotrimazole 1% W/W Dusting Powder
- Ornidazole 500mg Tablets
- Salicylic Acid Ip Sulphure Usp Soap
- Prednisolone 5 Mg Tablets
- Nimesulide 100 Mg Tablets
- Aceclofenac 100 Mg Serratiopeptidase 15 Mg Tablets
- Metoprolol Tartrate 50 Mg Sustain Release Tablets
- Doxylamine Succinate 10Mg Pyridoxine Hcl 10 Mg Tablets
- Glipezide 5 Mg Tablets
- Clotrimazole Vaginal 100 Mg Tablets
- Metronidazole 200 Mg Tablets
- Biotin 5mg Folic Acid 5mg Capsule
- Montelukast Sodium 10 Mg Tablets
- Pantoprazole 30 Mg Ondonsetron 4 Mg Tablets
- Aceclofenac Linseed Oil Methyl Salicylate Menthol Gel
- Dicyclomine 10 Mg Mefenamic Acid 250 Mg Tablets
- Drotaverine 80Mg Tablets
- Cefpodoxime Potasium Clavunate Dry Syrup
- Diclofenac Linceed Oil Methyl Salicylate Menthol Gel
- Ketoconazole Zinc Pyrithione Zpto Shampoo
- Gabapentin 600 Mg Tablets
- Meloxicam 15 Mg Tablets
- Azithromycin Suspension
- Beclomethasone Propionate Miconazole Neomycin Cream
- Cyproheptadine Hydrochloride Syrup
- Griseofulvin Tablets 125 Mg
- Levofloxacin Ornidazole Dry Syrup
- Fluocinolone Miconazole Neomycin Cream
- Povidone Iodine Ornidazole Ointment
- Ferrous Fumarate Folic Acid Vit B12 Zinc Sulphate Tablets
- Loperamide Hcl 2 Mg Capsule
- SERTACONAZOLE 2 % W/W CREAM
- Calamine With Vitamin E, Aloe Vera & Olive Oil Lotion
- MOMETASONE FUROATE CREAM
- Artemether Lumifentrin Dry Syrup
- Clobetasol Propionate Clotrimazole Neomycin Cream
- Amorolfine 0.25 % W/w Cream
- Erythromycin Estolate 250 Mg Tablets
- Salbutamol Sulphate Cholin Theophylline Menthol Syrup
- Etoricoxib 60 Mg Tablets
- Enalapril Maleate 10 Mg Tablets
- Sertraline Hydrochloride 25 Mg Tablets
- Doxycycline Dispersible 100Mg Tablets
- Piroxicam 20 Mg Tablets
- Febendazole - 150 MG TABLETS
- CLOBETASOL PROPIONATE CREAM
- Clotrimazole Vaginal Cream
- Clarithromycin 250 Mg Tablets
- Ibuprofen 200 Mg Tablets
- Carbonyl Iron Folic Acid Zinc Vitaminb12 Vitamin C Capsules
- Metronidazole 400mg Furazolidone Simethicone Tablets
- Diltiazem 60Mg Tablets
- Ciprofloxacin 500Mg Ornidazole 500Mg Tablets
- Enalapril Maleate 5Mg Tablets
- FEBUXOSTATE Tablets 80mg
- Betamethasone 0.5Mg Tablets
- Permethrin Soap
- Atorvastatin 10 Mg Tablets
- Acetazolamide 250Mg Sustain Release Tablets
- Chloroquine 500Mg Tablets
- Vitamin E With Aloe Vera Moisturising Soap
- Hydrocortisone 100 Mg Tablets
- Diclofenac Thiocolchicoside Capsules
- Ambroxol Guaiphenesin Terbutaline Sulphate Syrup
- Ondansetrone 8 Mg Tablets
- Paediatric Haemitinic Drops
- Stanozolol Usp Powder
- Betamethasone and Gentamycin Cream
- Bisacodyl 5 Mg Tablets
- Praziquantel Pyrantel Pamoate And Fenbendazole Tablet
- Benidipine Hydrochloride 8 Mg Tablets
- Venlafaxine 25mg Tablets
- Co-trimoxazole 120 Mg Tablets
- Acarbose 50 Mg Tablets
- Repaglinide 1 Mg Tablets
- Naproxen Sodium 275mg Tablets
- Pregabalin 75 Mg Methylcobalamin 750 Mcg Tablets
- Doxycycline Hcl 100 Mg Tablets
- Benidipine Hydrochloride 4Mg Tablets
- Azithromycin 250 Mg Tablets
- Acarbose Tablets 25 Mg
- Acyclovir Dispersible Tablets
- Active Pharmaceuticals Ingredients (API)
- Gallery
- Contact Us

Showroom
Active Pharmaceuticals Ingrediants (API) are used in a finished pharmaceutical product, intended to furnish pharmacological activity or to otherwise have direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease. They are the part of any drug that produces the intended effects.
Pharma Intermediates are manufactured using optimum grade raw materials and recent technology under the supervision of our dedicated and experienced experts. These intermediates are the drugs used as raw materials for the production of bulk drugs, or they can refer to a material produced during synthesis of an API.
Pharmaceutical Formulation is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form. This is defined as the process in which different chemical substances are combined to produce a final medicinal product.
Contact Details
NIKSAN PHARMACEUTICAL
Ankleshwar, Gujarat, India
Ankleshwar, Gujarat, India
- Plot No. 4706/03, Gidc Estate, SF-12, Shrinathji Arcade, Near Meghmani Chowkadi,Ankleshwar - 393002, Gujarat, India
- Phone :91--9537871777
- Email : info@niksanpharmaceutical.com
- Mr SANJAY PATEL (SALES DEPARTMENT)
- Mobile :+919537871777
- info@niksanpharmaceutical.com
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese