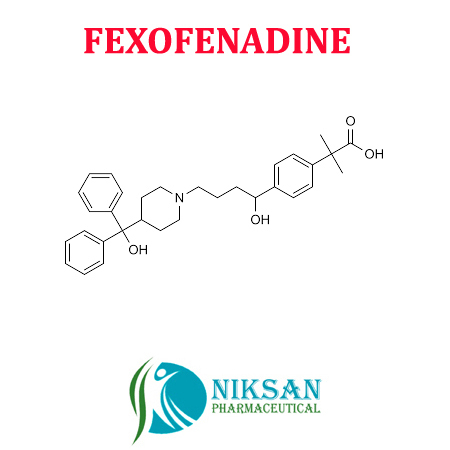
FEXOFENADINE
4500 INR/Kilograms
Product Details:
- Molecular Weight 501.68 g/mol Grams (g)
- HS Code 29420090
- Shelf Life 3 Years
- Molecular Formula C32H39NO4
- Storage Dry Place
- Medicine Name FEXOFENADINE
- Chemical Name FEXOFENADINE
- Click to View more
X
FEXOFENADINE Price And Quantity
- 4500 INR/Kilograms
- 5 Kilograms
- 4500.00 - 6000.00 INR/Kilograms
FEXOFENADINE Product Specifications
- FEXOFENADINE
- 83799-24-0
- FEXOFENADINE
- Powder
- 501.68 g/mol Grams (g)
- Fexofenadine belongs to the drug class called antihistamines. Fexofenadine used in the allergic treatment like watery eye, swollen skin, rash, itching or irritation. Fexofenadine prevents the allergic reaction by blocking the effects of histamine.
- Other
- White to Off-White Solid
- Dry Place
- C32H39NO4
- Medicine Grade
- 99 %
- 3 Years
- 29420090
FEXOFENADINE Trade Information
- INDIA
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Dadra and Nagar Haveli Chandigarh Himachal Pradesh Andaman and Nicobar Islands Uttarakhand Daman and Diu Lakshadweep Nagaland South India Central India North India East India West India Andhra Pradesh Assam Arunachal Pradesh Bihar Delhi Gujarat Goa Haryana Jammu and Kashmir Jharkhand Karnataka Kerala Madhya Pradesh Maharashtra Mizoram Meghalaya Manipur Odisha Punjab Pondicherry Rajasthan Sikkim Tamil Nadu Telangana Tripura Uttar Pradesh West Bengal Chhattisgarh All India
- WHO GMP,GMP,GLP,ISO
Product Description
HOW TO USE:Take medication directly by mouth 2 timesper day after 12 hours.If you use oral solution, kindly shake well beforeuse.Take your doctor™s advice before using the medication.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese