QUETIAPINE
Product Details:
- EINECS No 601-143-7
- Shelf Life 3 Years
- Molecular Weight 383.50 g/mol Grams (g)
- Particle Size 200-250 nm
- Taste Bitter
- Melting Point 171-178C
- Molecular Formula C21H25N3O2S
- Click to View more
QUETIAPINE Price And Quantity
- 100 Kilograms
QUETIAPINE Product Specifications
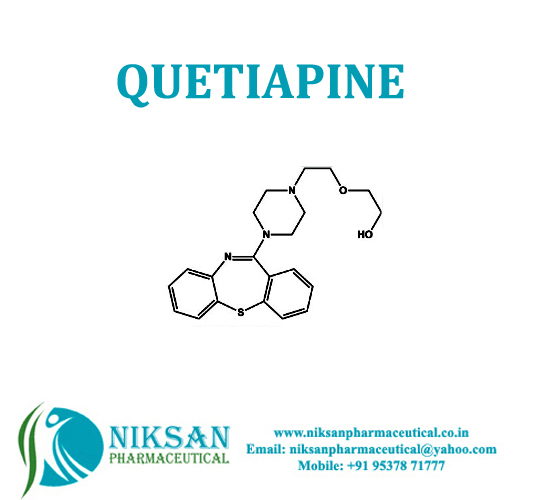
- 2-(2-(4-dibenzo[b,f]thiazepine-11-yl-1-piperazinyl)ethoxy)ethanol
- 601-143-7
- QUETIAPINE
- 3 Years
- 383.50 g/mol Grams (g)
- 200-250 nm
- 111974-69-7
- Other
- 99 %
- Bitter
- Quetiapine is an antipsychotic medicine which is used to treatment of schizophrenia in adults and children who are at least 13 years old.
- 171-178C
- Powder
- C21H25N3O2S
- 556.5C at 760 mmHg
- 300490
- Other
- Other
- Other
- White to Off-White Solid
- less than 0.001%
- 94.3 mg/mL at pH 1 to 2.37 mg/mL at pH 9
- moderately soluble in water
- C21H25N3O2S
- not more than 0.5% when dried at 105C
QUETIAPINE Trade Information
- Nhava Sheva or Mumbai
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- All India, South India, Central India, West India, North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Jharkhand, Odisha, Punjab, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Chhattisgarh, Haryana, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal
- GMP, FDCA
Product Description
Niksan Pharmaceutical is top leading manufacturer, supplier,exporter and trader in Indian states and also in worlds other countries. Quetiapine is the best product ofNiksan Pharmaceutical which exporting as a form of API and finishedformulations.
Niksan Pharmaceutical provides API and finished formulation of Quetiapine in many Indian states likeMaharashtra, Odisha, Uttar Pradesh, Delhi, Rajasthan, Madhya Pradesh, WestBengal, Bihar, Himachal Pradesh, Andhra Pradesh, Tamil Nadu, Telangana, Kerala,Karnataka, Gujarat, Jammu & Kashmir, Chandigarh, Punjab, Uttarakhand,Chhattisgarh, Haryana, Assam and many other Indian states.
Niksan Pharmaceutical is also huge exporter of the API andfinished pharmaceutical products of Quetiapinein many countries for years. The countries where we exporting are India, Nepal,Japan, United Arab Emirates, Hong Kong, Taiwan, India, France , Canada,Bangladesh, Poland, Pakistan, Thailand, Netherlands, Turkey, Vietnam,Philippines, Iran, Canada Ukraine, Bolivia, Bangladesh, Philippines, Thailand,Saudi Arabia, Italy, Australia, Canada, United States, United Kingdom, Mexico,Indonesia and many other countries.
Quetiapine is an antipsychotic medicine which is used to treatment ofschizophrenia in adults and children who are at least 13 years old.
Quetiapine is also used to treatment of bipolar disorder in 10 year oldchildren and adults.
SYNONYMS: Quetiapina, Qutiapine, Quetiapine, Quetiapinum
IUPAC NAME:2-[2-(4-{2-thia-9-azatricyclopentadeca-1(15),3,5,7,9,11,13-heptaen-10-yl}piperazin-1-yl)ethoxy]ethan-1-ol
CAS NO: 111974-69-7
FORMULA: C21H25N3O2S
MOLECULAR MASS: 383.50 g/mol
STORAGE OF Quetiapine: Store it incool and dry place, away from moisture and direct light. Do not store this medicine in bathroom or anyhumidly place.
APPLICATIONS OF Quetiapine: Quetiapine is an anti-psychotic drug which is used to treatment of bipolar disorders, sudden episodes of mania or depression and mentalcondition. It is also use as sleeping drug.
HOW TO USE: Quetiapine is taken by mouth without food or lightmeal. Do not crush the tablet. Generally you can take 3 tablets in one day.
HOW QUETIAPINE WORKS: Quetiapineworks in human brain to treat schizophrenia. Quetiapine also improve your mood,thinking and behaviour.
CONTRAINDICATIONS:If you have a breast cancer and diabetes kindlyavoid this medication. If you have a low amount of potassium and magnesium inyour blood do not take this medication. If you have a slow heart beat and liverproblems kindly avoid Quetiapine. Kindlyavoid this medication if you are allergic to it.
PHARMACOKINETIKS OF Quetiapine:Quetiapine absorbed rapidly and well after oraladministration. Half life of Quetiapine is about 6 to 7 hours. Quetiapine ismainly metabolized in liver. Time of peak plasma concentration of Quetiapine is1.5 hour after oral administration. 73% excretion is done by urine and 20%excreted via feces.
SIDE EFFECTS OF Quetiapine: Some common sideeffects are upset stomach, nausea, vomiting, drowsiness, behaviour changes,constipation and stomach pain. Other side effect of Quetiapine is tiredness,headache, and trouble in sleeping, dry mouth, sore throat and weight gain.
PRECAUTION: Before using Quetiapineshare your medical history with your doctor. If you are allergic to Quetiapinekindly ask your doctor. This drug may make you dizzy so kindly avoid alcoholand cannabis. Do not drive.
CDSCO APPROVAL: Quetiapine (As fumarate) SR 400mg tablet(additional strength) is approved by CDSCO in India in 05.03.2007.
QuetiapineSR Tablets 50mg (additional strength) is approved by CDSCO in India in 10.04.2007
Quetiapinefumerate tablet is approved by CDSCO in India in 03.06.2002.
Quetiapinefumarate SR tablet is approved by CDSCO in India in 21.02.2008.
QuetiapineS.R tablet 200/300mg is approved by CDSCO in India in 01.08.2006.
FORMULATIONS AVAILABLE IN MARKET:
Quetiapine50 MG tablets
Quetiapine25 MG tablets
Quetiapine100 MG tablets
Quetiapine200 MG tablets
Quetiapine300 MG tablets
Quetiapine400 MG tablets
Quetiapine(As fumerate) SR 400mg tablets (additional strength)
QuetiapineSR Tablets 50mg (additional strength)
Quetiapinefumerate tablets
Note: Product protected by valid patents are not offered for sale incountries where such patents are still valid and its liability is at BuyersRisk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese