CELECOXIB
Product Details:
- Taste Other
- Molecular Weight 381.37 g/mol Grams (g)
- Structural Formula C17H14F3N3O2S
- HS Code 29420090
- Loss on Drying 0.5% to 1.0%
- Particle Size submicron (nanoparticles) to micrometers
- EINECS No 685-962-5
- Click to View more
CELECOXIB Price And Quantity
- 10 Kilograms
CELECOXIB Product Specifications
- 29420090
- Other
- 381.37 g/mol Grams (g)
- White to Off-White Solid
- Medicine Grade
- CELECOXIB
- C17H14F3N3O2S
- 99 %
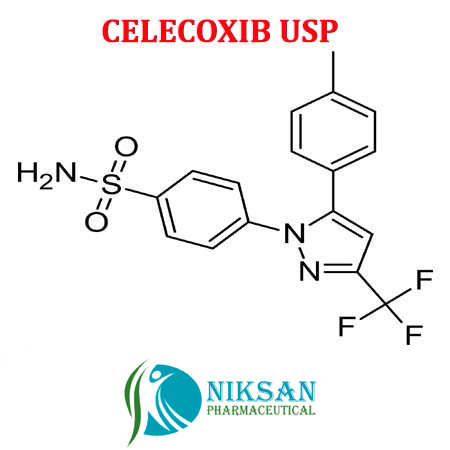
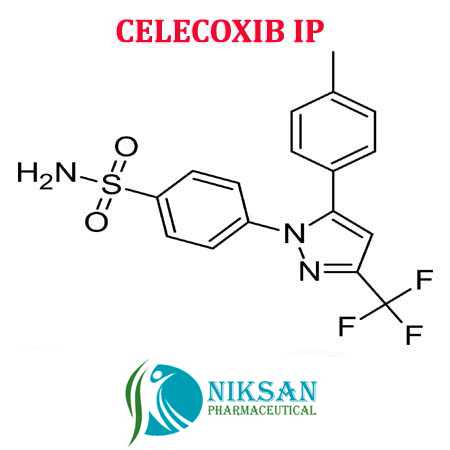
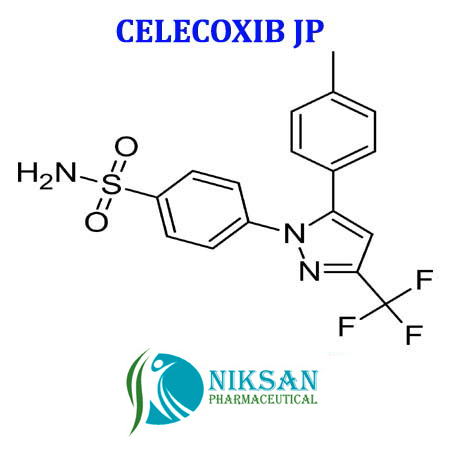
- 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide
- submicron (nanoparticles) to micrometers
- 0.5% to 1.0%
- 3 Years
- C17H14F3N3O2S
- Room Temperature
- Other
- Powder
- 685-962-5
- poor solubility in water, with reported values ranging from 3 to 7 g/mL at 37C and pH 7
- pH 7 and 40C.
- less than 0.002%
- 524.3 60.0 C at 760 mmHg
- 169590-42-5
- Other
- Celecoxib belongs to the non-steroidal anti-inflammatory drug. Celecoxib generally used to treat the pain in the gout, rheumatoid arthritis, joint pain, osteoarthritis, inflammation in joints and kidney failure.
- 156C to 165C
CELECOXIB Trade Information
- SAHAR AIR CARGO
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- All India
- FDCA, GMP, GLP AND ISO
Product Description
is one of the leading Manufacturer, Exporter, Distributor, andSupplier of Celecoxibin Ankleshwar, Gujarat, India. To understandthe largest accomplishment of clients, we present these products at veryrealistic price ranges. Niksan Pharmaceutical and Niksan group companies arenowadays one of the largest manufacturers and exporters of CelecoxibAPI and also finished formulations.
NiksanPharmaceutical and Niksan group companiesare the very well-known manufacturers, suppliers and distributors of Celecoxibproducts.
Niksan Pharmaceutical providesAPI and finished formulation of Celecoxib in manyIndian states like Himachal Pradesh, Andhra Pradesh, Tamil Nadu, Telangana,Kerala, Karnataka, Gujarat, Jammu & Kashmir, Chandigarh, Punjab,Uttarakhand, Chhattisgarh, Haryana, Maharashtra, Odisha, Uttar Pradesh, Delhi,Rajasthan, Madhya Pradesh, West Bengal, Bihar, Assam and many other Indianstates.
NiksanPharmaceutical are manufacturing and exporting very large quantity of Celecoxib in countries like Zimbabwe,Peru, Philippines, Chile, Dominican Republic, Mexico,New Zealand, Guatemala, Honduras, Costa Rica,SriLanka, Ghana, Qatar, Ecuador,Vietnam, ElSalvador, Colombia, Bolivia, Australia,Hong Kong, UnitedArab Emirates, Panama, Kenya,Singapore, Jordan,Canada, Malaysia,Iraq, Iran,United States, Nigeria, Pakistan, Romania, SouthKorea, Saudi Arabia, Germany, Netherlands,Taiwan, Spain,Thailand, Switzerland,South Africa, Portugal, Israel, Ireland, Algeria,Egypt, Belgium,Sweden, UnitedKingdom, Venezuela, Finland, Indonesia,Austria, Italy,China, Argentina,France,Brazil, Japan, Poland and many othercountries.
Celecoxib belongs to the non-steroidal anti-inflammatorydrug. Celecoxib generally used to treat the pain in the gout, rheumatoidarthritis, joint pain, osteoarthritis, inflammation in joints and kidneyfailure.
Celecoxib is cox- selective inhibitor.
SYNONYMS: Celecoxib, Clcoxib, Celecoxibum.
IUPAC NAME: 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzene-1-sulfonamide
CAS NO: 169590-42-5
FORMULA: C17H14F3N3O2S
MOLECULAR MASS: 381.37 g/mol
STORAGE CONDITION: Store in room temperature, away from heat anddirect light. Do not store in kitchen or bathroom. Keep away from children andpet.
HOW TO USE: Take medication once or twice a day by orallywith water. To avoid the chance of stomach pain or stomach upset takemedication with food. Take your doctorsadvice if you have any confusion.
HOW CELECOXIB WORKS: Celecoxib give relief in, rheumatoid arthritis,joint pain, osteoarthritis, inflammation in joints, pain in the gout, byinhibiting the actions of COX-2 enzyme. Celecoxib also used to treat swellingin joints.
PHARMACOKINETICS OF CELECOXIB: Celecoxib rapidly absorbed in GI track afteroral administration. It takes 1-2 hourstoreach the max plasma concentration. Almost97% of Celecoxib binds with albumin in body. The half life of Celecoxib is 11hours in the normal person. The primary elimination of Celecoxib done byhepatic metabolism, 57% of Celecoxib eliminated by faces and the remainingresidues are excreted by urination.
SIDE EFFECTS OF CELECOXIB: Normally gas and stomach upset are the sideeffects seen in patients. The several side effects like headache, swelling ofcalf, kidney problems, problem in urination, yellow eyes, stomach pain. Getmedical attention if you see side effects like black urine, swelling of limbs,stomach problem or yellowing of skin/eyes. There are some rare side effectslike itching, swelling, dizziness, irritation, trouble in breathing also seenin patients.
PRECAUTIONS: Kindly tell you doctor if you have allergicreactions towards the Celecoxib medication. Do not take Celecoxib with theaspirin and other NSAID products.
Kindly avoid taking Celecoxib medicine if you have problemlike liver problem, heart problem, kidney problem, stroke, blood pressureproblem, ulcers. Take your doctors advice if you are pregnant or in lactationperiod. Use of alcohol or tobacco may cause stomach bleeding.
CDSCO APPROVAL: Celecoxib capsule approved by CDSCO in Indian in 22-02-2000,Celecoxib 100/200mg +Diacerein 50/50mg approved by CDSCO in Indian in19.07.2010,
Celecoxibcapsule approved by CDSCO in Indian in 15.02.2002,
CelecoxibMouth Dissolving Tablet 50/100/200 mg approved by CDSCO in Indian in25.02.2010,
Celecoxibinjection approved by CDSCO in Indian in 2004-October, Celecoxib injectionapproved by CDSCO in Indian in 27.10.2004.
FORMULATION AVAILABLE IN MARKET:
Celecoxib100mg +Diacerein 50mg capsules
Celecoxib200mg +Diacerein 50mg capsules
Celecoxib50 mg Mouth Dissolving Tablets
Celecoxib100 mg Mouth Dissolving Tablets
Celecoxib200 mg Mouth Dissolving Tablets
Celecoxibinjection
Celecoxib200mg capsules
Celecoxib100mg capsules
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Efficient Pain Relief for Joint Disorders
Celecoxib offers effective relief from chronic pain and inflammation caused by gout, rheumatoid arthritis, osteoarthritis, and other joint diseases. By targeting inflammation at its source, it helps patients regain mobility and comfort, making daily activities more manageable. Its reliable formulation meets stringent quality standards, ensuring consistent results for long-term and acute pain management.
Stringent Manufacturing and Quality Standards
As a leading supplier, manufacturer, and exporter of Celecoxib, Niksan Pharmaceutical follows GMP practices and maintains a ready DMF. Each unit is produced under strict pharmaceutical norms, ensuring high purity and precise chemical composition. Rigorous testing for heavy metals and loss on drying, alongside stable storage requirements, guarantees sustained efficacy and safety throughout its shelf life.
Guidelines for Safe Usage and Storage
Celecoxib tablets and capsules should be taken orally with water, once or twice a day as prescribed. It is essential to keep the medication stored in a well-closed container at room temperature, avoiding exposure to heat and light. Proper handling preserves its potency and prevents degradation, contributing to optimal therapeutic benefits for patients managing chronic pain conditions.
FAQs of CELECOXIB:
Q: How should Celecoxib be stored to maintain its effectiveness?
A: Celecoxib should be stored in a well-closed container at room temperature, away from heat and direct light. Keeping it in a dry place and ensuring the lid is tightly sealed helps preserve its potency and extend its shelf life up to three years.Q: What is the recommended way to take Celecoxib tablets or capsules?
A: The medication is to be taken orally, once or twice daily with water, as directed by a healthcare provider. It is best taken at the same time each day to maintain consistent levels in the body for effective pain management.Q: What conditions does Celecoxib help treat?
A: Celecoxib is primarily used to relieve pain and inflammation in conditions such as gout, rheumatoid arthritis, osteoarthritis, joint pain, and inflammation related to kidney failure. It assists patients in managing chronic discomfort and improving mobility.Q: What benefits does Celecoxib offer for arthritis patients?
A: As an NSAID, Celecoxib reduces pain, swelling, and inflammation in the joints, enabling patients with arthritis to enjoy greater comfort and improved joint function. Its targeted action on inflammation helps restore mobility and enhances quality of life.Q: How is Celecoxib manufactured and ensured for quality?
A: Celecoxib is produced by Niksan Pharmaceutical following GMP guidelines and is exported globally. Each batch undergoes rigorous quality checks, including tests for heavy metals, purity, and stability, guaranteeing high-quality medicine-grade raw material suitable for pharmaceutical use.Q: What is the chemical profile and appearance of Celecoxib?
A: Celecoxib has the molecular formula C17H14F3N3O2S, a purity of 99%, and appears as a white to off-white solid powder. It has a molecular weight of 381.37 g/mol, a melting point range of 156C to 165C, and displays poor solubility in water.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese