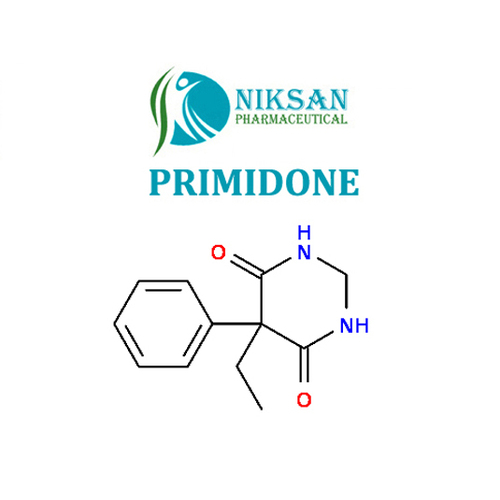
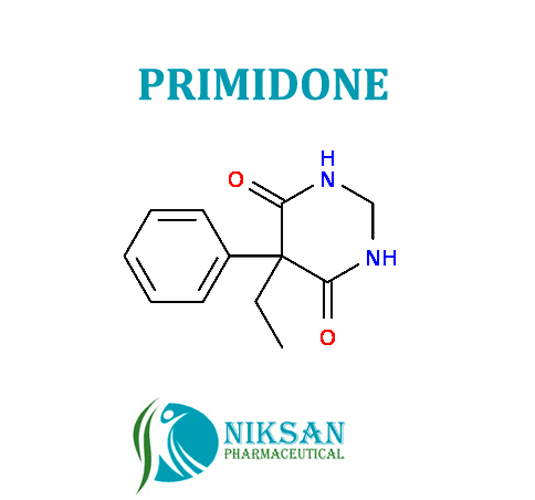
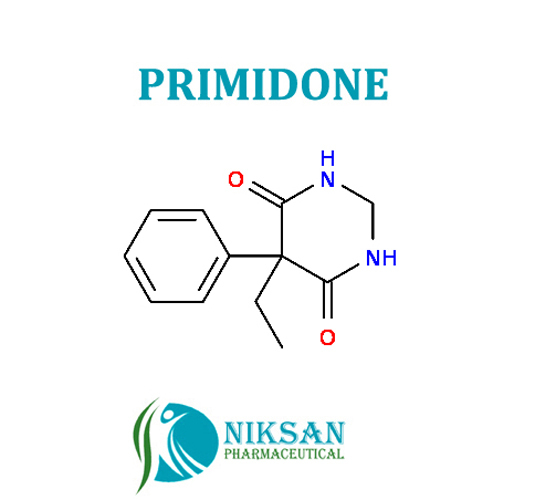
PRIMIDONE
Product Details:
- Solubility practically insoluble in water (1/2000), slightly soluble in alcohol (1/200), and soluble in alkaline solutions
- Smell Other
- Shelf Life 3 Years
- Particle Size 149.9 to 188.8 nm
- Melting Point 281C
- Storage Room Temperature
- Boiling point 520.7 C
- Click to View more
PRIMIDONE Price And Quantity
- 1 Kilograms
PRIMIDONE Product Specifications
- practically insoluble in water (1/2000), slightly soluble in alcohol (1/200), and soluble in alkaline solutions
- Other
- Other
- Powder
- 281C
- Room Temperature
- 520.7 C
- Primidone is one type of antiepileptic medication developed to treat seizures, commonly for partial and generalized seizures.
- 3 Years
- 149.9 to 188.8 nm
- White to Off-White Solid
- Medicine Grade
- PRIMIDONE
- 99 %
- 125-33-7
- C12H14N2O2
- 204-737-0
- Other
- Lead
- PRIMIDONE
- C12H14N2O2
- 218.25 g/mol Grams (g)
- NA
- Not more than 0.5%
- 2933.59
PRIMIDONE Trade Information
- INDIA
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- All India, South India, Central India, West India, North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Jharkhand, Odisha, Punjab, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Chhattisgarh, Haryana, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal
- GMP, FDCA
Product Description
SYNONYMS: 2-deoxyphenobarbital, Primidon, Primidona, Primidone, Primidonum
IUPAC NAME: 5-ethyl-5-phenyl-1,3-diazinane-4,6-dione
CAS NO: 125-33-7
FORMULA: C12H14N2O2
MOLECULARMASS:218.25 g/mol
STORAGE OF PRIMIDONE : Store the medicine in a closed container at roomtemperature, away from heat, moisture, and direct light. Keep from freezing.
APPLICATIONSOF PRIMIDONE:Primidone is used alone or with other medications to control certain types of seizures.Primidone is in a class of medications called anticonvulsants. It works bydecreasing abnormal electrical activity in the brain.
It is usually taken 3 to 4 times a day.
PRIMIDONE WORKS: Primidone isused alone or with other medications to control certain types of seizures.Primidone is in a class of medications called anticonvulsants. It worksby decreasing abnormal electrical activity in thebrain.
PRIMIDONE: Primidone is contraindicated in patients who are known to behypersensitive to barbituric acid derivatives. Contraindications to primidoneinclude patients with a history of porphyria. By inducing the enzymesresponsible for porphyrin synthesis, barbiturates may worsen acute porphyria.
PHARMACOKINETICSOF PRIMIDONE: Primidone is well absorbed and distributed throughout the body. Maximum plasma concentration is reached in about 2 h afteradministration of a 250 mg tablet. Primidone is metabolized in the liver toactive metabolites: phenobarbital and phenylethylmalonic acid (PEMA).
SIDE EFFECTS OF PRIMIDONE: The most common sideeffect of primidone therapy is sedation and drowsiness. Ataxia, diplopia,and nystagmus occur at the initiation of treatment. Other adverse reactionsinclude dizziness, vertigo, epigastric pain, megaloblastic anemia, respiratorydepression, polyuria, skin rash, facial edema.
PRECAUTIONS: Check with your doctor before taking any of the above while you areusing this medicine. Primidone may cause some people to become dizzy,lightheaded, drowsy, or less alert than they are normally. Even if taken atbedtime, it may cause some people to feel drowsy or less alert on arising.
CDSCO APPROVAL:
Primidone tablet 50mg are approved by CDSCO in India in 15.04.2019
FORMULATION AVAILABLE IN MARKET:
Primidone12.5mg tablet
Primidone25mg tablet
Primidone50mg tablet
Primidone100mg tablet
Primidone250mg tablet
Note: Productprotected by valid patents are not offered for sale in countries where suchpatents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese