ORN IDAZOLE
Product Details:
- Storage Room Temperature
- EINECS No 240-826-0
- Molecular Formula C7H10ClN3O3
- Loss on Drying typically below 0.5%
- Shelf Life 3 Years
- Smell Other
- HS Code 29420090
- Click to View more
ORN IDAZOLE Price And Quantity
- 5 Kilograms
- 2800 INR/Kilograms
ORN IDAZOLE Product Specifications
- Pharmaceutical Intermediates
- Medicine Grade
- Room Temperature
- 240-826-0
- White to Off-White Solid
- C7H10ClN3O3
- typically below 0.5%
- Powder
- 3 Years
- Ornidazole
- Other
- 16773-42-5
- ORNIDAZOLE
- 29420090
- nanometers to millimeters
- 85 to 93C
- Ornidazole belongs to the anti-protozoal class of medicine. Ornidazole works by killing the protozoa and bacteria and prevents the infections caused by them.
- 443.2 C
- 99 %
- 219.625 g/mol Grams (g)
- C7H10ClN3O3
- pH 6.5 TO pH 7.4
- NMT 20 ppm
- freely soluble in ethanol and chloroform, and sparingly soluble in water
- Other
ORN IDAZOLE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Week
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
belongs to the anti-protozoal class ofmedicine. Ornidazole works by killing the protozoa and bacteria and preventsthe infections caused by them.
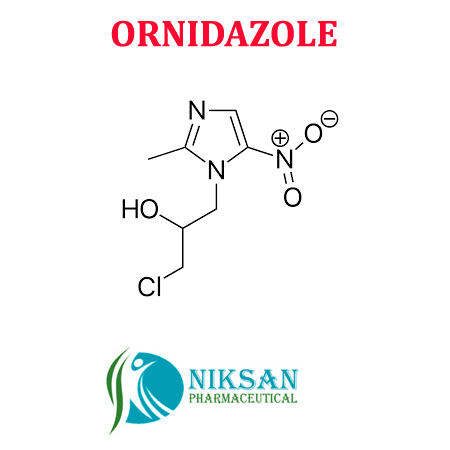
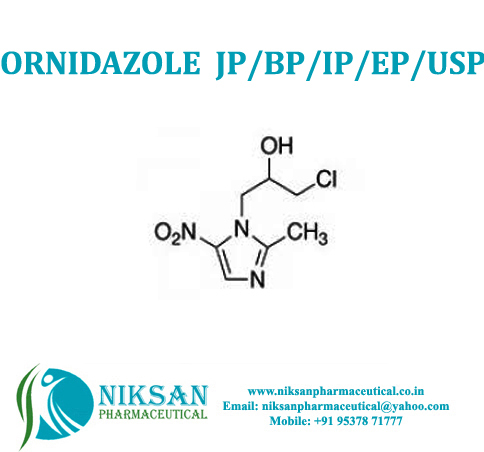
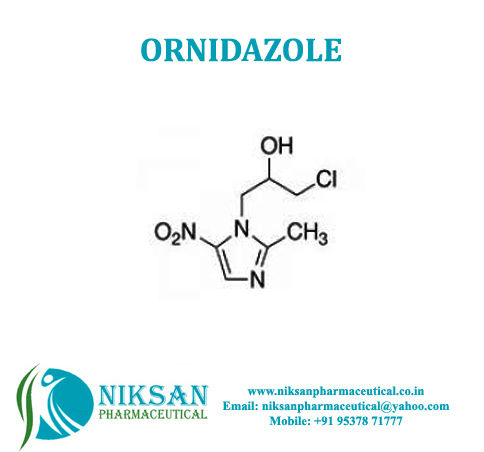
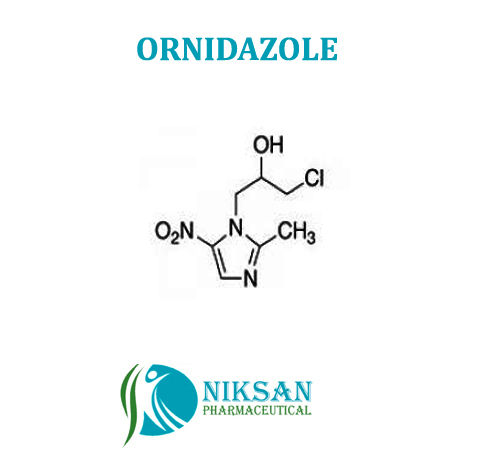
IUPAC NAME: 1-chloro-3-(2-methyl-5-nitro-1H-imidazol-1-yl)propan-2-ol
CAS NO: 16773-42-5
FORMULA: C7H10ClN3O3
MOLECULAR MASS:219.625 g/mol
STORAGE CONDITIONS: Store in cool and dry place, away from heat andlight. Do not put in bathroom or ant misty place. Keep away from children andpets.
HOW TO USE: Take medication twice a daywith or without water. Take advice of your doctor if you have any confusion. Ifyou feel stomach upset take medication with food or milk.
HOW ORNIDAZOLE WORKS: Ornidazole works by killing the protozoa andbacteria inside the body. Ornidazole prevents bacterial ell wall synthesis andthis Ornidazole kills bacteria and amoeba.
PHARMACOKINETICS:Ornidazole absorbed rapidly after the oral administration. Ornidazolehave bioavaibility around 90%. The half-life of Ornidazole about 3 hours, TheOrnidazole excreted through urination.
SIDE EFFECTS: The common side effects ofOrnidazole are headache, nausea, vomiting and dryness of mouth. If you see sideeffects like stomach pain, dizziness, fainting contact your doctor.
PRECAUTIONS: Tell your doctor if you have any disease likeliver problem, heart problem or kidney problem. Do not take alcoholic productswhile taking the medicine. Do not take Ornidazolemedicine if you are in pregnancy or in lactation period.
CDSCO APPROVAL:Ciprofloxacin+ Ornidazole tab approved by CDSCO in India in 03.02.2003
Ofloxacin 2mg + Ornidazole 5 mg per ml Infusion approved by CDSCOin India in 17.08.2009
Cefixime + Ornidazole tablets approved by CDSCO in India in05.12.2005
Ornidazole tablet/infusion/bulk approved by CDSCO in India in13.01.1997
Ornidazole IP 1 % w/w + Povidone Iodine IP (0.5% w/w availableIodine) 5% w/w Ointment approved by CDSCO in India in 03.01.2012
Ornidazole vaginal tabs. approved by CDSCO in India in 04.07.2001
Gatifloxacin (200mg) + Ornidazole (500mg) tablet approved by CDSCOin India in 06.06.2005
Ofloxacin (200mg) + Ornidazole (500mg) approved by CDSCO in Indiain 19.01.2005
Comb pack of 1 bottle of ciprofloxacin Injection (200mg/100ml)& 1 bottle of Ornidazole injection 500mg/100ml) approved by CDSCO in Indiain 20.04.2007
FORMULATIONS AVAILABLE IN MARKET:
Ciprofloxacin + Ornidazole tablets
Ornidazole IP 1 % w/w + Povidone Iodine IP 5% w/w OintmentOfloxacin 2mg + Ornidazole 5 mg per ml Infusion
Ornidazole vaginal tablets
Gatifloxacin (200mg) + Ornidazole (500mg) tablets
Ofloxacin (200mg) + Ornidazole (500mg) tablets
Comb pack of 1 bottle of ciprofloxacin Injection (200mg/100ml)& 1 bottle of Ornidazole injection 500mg/100ml)
Ornidazole 10mg tablets
Ornidazole 500mg tablets
Miconazole 2%w/w + Ornidazole 2% w/w cream
Ornidazole IP 1 % w/w + Povidone Iodine IP 5% w/w cream
Ornidazole 500mg/100ml solutions
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Broad Spectrum Anti-Bacterial Solution
Ornidazoles robust formulation makes it an effective solution against a wide range of bacterial and protozoal infections. By disrupting the DNA synthesis of pathogens, it directly targets the root of the infection, delivering reliable relief and reducing the risk of recurrence.
Versatile Dosage Forms for Comprehensive Treatment
This pharmaceutical intermediate can be administered as tablets, creams, or injections, offering flexible treatment options tailored to specific infections and patient needs. The diverse dosage forms help in targeting infections efficiently, whether systemic or localized.
Optimal Storage and Longevity
With a shelf life of up to 3 years, Ornidazole retains its potency when stored in a cool, dry place, away from heat and light. Adhering to recommended storage guidelines ensures the medication remains effective throughout its use.
FAQs of ORNIDAZOLE:
Q: How should Ornidazole be stored to maintain its effectiveness?
A: Store Ornidazole in a cool, dry place, away from heat and direct light. Do not keep it in the bathroom or any humid environment to prevent degradation and maintain its shelf life.Q: What is the correct way to take Ornidazole?
A: Ornidazole should be taken twice daily with or without water, as directed by your healthcare provider. The specific dosage and form (tablet, cream, or injection) will depend on your condition and the recommendation of a medical professional.Q: What types of infections can Ornidazole be used to treat?
A: Ornidazole is effective against protozoal and bacterial infections, such as amoebiasis, giardiasis, and trichomoniasis. It works by killing the causative microorganisms, thereby preventing and treating the infection.Q: Is Ornidazole available in different forms for use?
A: Yes, Ornidazole is available in various dosage forms including tablets, topical creams, and injections, making it suitable for both systemic and localized infections.Q: What are the main benefits of using Ornidazole?
A: Ornidazole provides rapid and efficient eradication of specific protozoa and bacteria, reduces the recurrence of infections, and comes in different dosage forms to suit patient and clinical needs.Q: Can Ornidazole be used by anyone for bacterial infections?
A: Ornidazole should only be used under medical supervision, as your healthcare provider will determine if its appropriate for your particular infection and overall health situation.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese