MO XIFLOXACIN HCL
Product Details:
- Smell Other
- Melting Point 238-250C
- Storage Room Temperature
- Heavy Metal (%) NA
- HS Code 30049039
- Solubility moderate solubility in water, with a solubility of approximately 2.9 mg/mL at 25C
- Loss on Drying NA
- Click to View more
MO XIFLOXACIN HCL Price And Quantity
- 5 Kilograms
- 12500.00 INR/Kilograms
MO XIFLOXACIN HCL Product Specifications
- NA
- 30049039
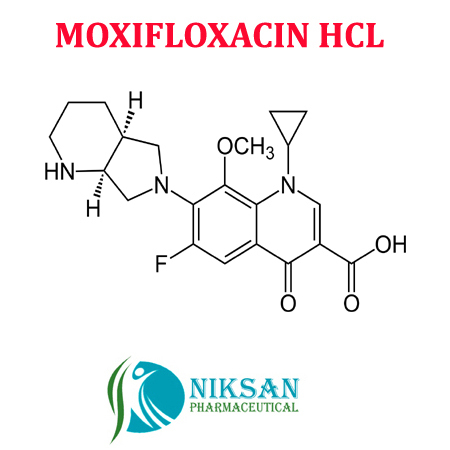
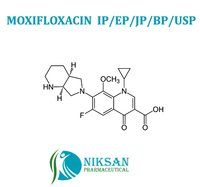
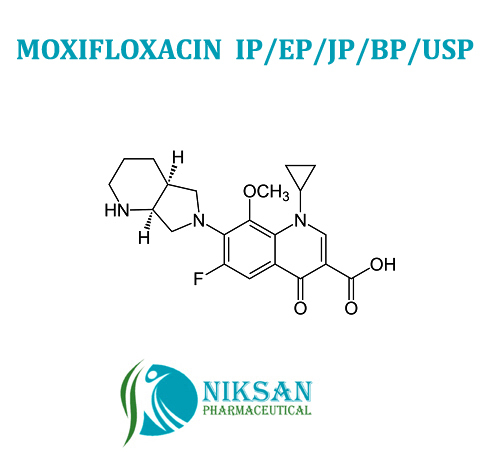
- 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid hydrochloride
- Other
- Industrial Grade
- 99%
- 238-250C
- Room Temperature
- 0.6 to 8.0 m
- yellow to yellow crystalline powder or solid
- Other
- Powder
- moderate solubility in water, with a solubility of approximately 2.9 mg/mL at 25C
- NA
- C21H24FN3O4HCl
- 636C
- 1.26.8
- Other
- 186826-86-8
- 3 Years
- 437.89 Grams (g)
- C21H24FN3O4HCl
- Moxifloxacin HCL is one type of antibiotic. Moxifloxacin HCL belongs to the class called fluoroquinolone. Moxifloxacin HCL gives antibiotic activity by killing the bacteria. Moxifloxacin HCL used in the treatment of pneumonia, tuberculosis, conjunctivitis.
- MOXIFLOXACIN HCL
MO XIFLOXACIN HCL Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
NiksanPharmaceutical is worlds leadingmanufacturer, trader, exporter and supplier of the Moxifloxacin HCL API and Moxifloxacin HCL formulations. Niksan Pharmaceuticalmanufactures large quantity of Moxifloxacin HCL API and finished products inAnkleshwar, Gujarat, India. Niksan Pharmaceutical and Niksan group companiesare the largest manufacturers and suppliers of Moxifloxacin HCL products.
NiksanPharmaceutical exporting very bigquantity of the fine quality products of Moxifloxacin HCL in all overworld for many years in a countries like Canada, USA, Romania, Finland,Malaysia, Netherland, South Africa, Brazil, Egypt, Singapore, Jordan, Lebanon,Israel, Vietnam, Bangladesh, Paraguay, Argentina, Dominican Republic, Sudan,Hong Kong, Seychelles, Algeria, Iran, Uruguay, Russia, Thailand, Afghanistan,Latvia, Lithuania, UnitedArab Emirates, Seychelles, Peru, Switzerland, Tunisia, France, Hungary,Finland, Turkey, Pakistan, Rwanda, South Africa, Denmark, Malawi, Croatia,Slovenia, Ireland, Zambia, Cyprus, Nigeria, Uzbekistan, Cameroon, Netherlands,Azerbaijan, Venezuela, Morocco, Cote Ivories, Lucia, South Korea, Congo,Philippines, Colombia Sweden, Hungary, Mauritius, Vanuatu, Malta, Kazakistan,Slovenia, Bolivia, Japan, Uganda, Australia and many more countries.
NiksanPharmaceutical also supplieslarge quantity of MoxifloxacinHCL products in Indian stateslike Gujarat, Haryana, Rajasthan, Madhya Pradesh, Kerala, Tamilnadu, Delhi,Bihar, Uttar Pradesh, Assam, Goa, Hyderabad, Telangana, Mizoram, Sikkim etc.
MoxifloxacinHCL is one type ofantibiotic. Moxifloxacin HCL belongs to the class called fluoroquinolone.Moxifloxacin HCL gives antibiotic activity by killing the bacteria.Moxifloxacin HCL used in the treatment of pneumonia, tuberculosis, conjunctivitis. Moxifloxacin HCL available in tablet, creams, ointments and capsules.
SYNONYMS: Moxifloxacin HCL, Moxifloxacino HCL
IUPACNAME: 1-Cyclopropyl-6-fluoro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydro-3-quinolinecarboxylicacid hydrochloride
CAS NO: 12-8039
MOLECULAR FORMULA: C21H25ClFN3O4
MOLECULAT MASS: 437.89 G/MOL
STORAGE CONDITIONS: Store in cool and dry place, away from direct sun light and heat. Donot store medicine in bathroom or any humid place. Keep away from children andpets.
HOW TO USE: Take medicine orally with or without food once per day. Wash eyes andremove the lance before using Moxifloxacinophthalmic solution. Do not consume the ophthalmic solution. Take your doctorsadvice if you have any confusion related to the medicine.
HOW MOXIFLOXACINHCL WORKS: Moxifloxacin inhibits thetopoisomerase II and topoisomerase IV enzyme. By inhibiting these enzymesMoxifloxacin inhibits the replication of DNA. And also inhibits the celldivision of the bacteria.
PHARMACOKINETICS: Moxifloxacin absorbed rapidly after oraladministration having bioavaibility of 90%. Almost 50% of drug binds withplasma proteins. Half-life of Moxifloxacin is between 11.5-15.6 hours.Approximately 45% of Moxifloxacin drug eliminated through urination and faces.
SIDE EFFECTSOF MOXIFLOXACIN: The common side effectsof Moxifloxacin are nausea, vomiting, headache, insomnia, weakness, dizzinessare seen in patients. If you see signs of kidney problem, liver problem, GIproblem like bleeding, stomach pain, abdominal discomfort, dark urine seekimmediate medical attention. Moxifloxacin also cause some intestinal problemlike bloody stool, abdominal pain, diarrhoea, cramping in intestine.
PRECAUTION: Tell your doctor if you have allergic reaction toMoxifloxacin medicine. If you have problem like liver problem, heart problem,depression, brain problem like Alzheimers, blood vessels problem, mooddisorders, brain tumours, or any blood pressure problem tell your doctor beforetaking the medication. Moxifloxacin can increase the heart problems so kindlyavoid the medicine if you have any heart related problems.
CDSCO APPROVAL: Moxifloxacin (0.5%) +Dexamethasone Phosphate (0.1%) Eye dropsapproved by CDSCO in India in 24.11.2005,
Moxifloxacin HCL (0.5%) +Ketorolac Tromethamine 0.5% Ophthalmic Solution approved by CDSCO in India in11.07.2007,
Moxifloxacin tab. approvedby CDSCO in India in 27.12.2001,
Difluprednate 0.05% w/v +Moxifloxacin 0.5% w/v Eye Drops app rovedby CDSCO in India in 22.09.2011
Moxifloxacin intravenousinfusion approved by CDSCO in India in 13.02.2002
Moxifloxacin HCl (5mg) +Prednisolone Acetate (10mg) per ml. (eyedro ps) approved by CDSCO in India in16.10.2007
Ketorolac Tromethamine 0.4%w/v + Moxifloxacin 0.5% w/v ophthalmicsol ution approved by CDSCO in India in 21.09.2010
Loteprednol etabonate 0.5%w/v + Moxifloxacin 0.5% w/v ophthalmic Suspension approved by CDSCO in India in01.12.2009
Moxifloxacin ophthalmicsolution approved by CDSCO in India in 19.10.2007
Moxifloxacin eye ointmentapproved by CDSCO in India in 01.10.2008
Moxifloxacin HCl approvedby CDSCO in India in 06.06.2001
Moxifloxacin HCl BP 0.5%w/v + Bromfenac Sodium 0.09% w/v Eye drop approved by CDSCO in India in05.01.2011
Moxifloxacin ophthalmicsolution 0.5% approved by CDSCO in India in 27.08.2004
Moxifloxacin Eye Ointment(5mg/gm) approved by CDSCO in India in 06.02.2006
Cefixime (SR) 400mg +Moxifloxacin (SR) 400 mg Tablets approved by CDSCO in India in 08.08.2011
FORMULATIONS AVAILABLE INMARKET:
Moxifloxacin (0.5%) +Dexamethasone Phosphate (0.1%) Eye drops
Moxifloxacin HCL (0.5%) +Ketorolac Tromethamine 0.5% OphthalmicSolution
Moxifloxacin tablets
Difluprednate 0.05% w/v +Moxifloxacin 0.5% w/v Eye Drops
Moxifloxacin intravenousinfusion
Moxifloxacin HCl (5mg) +Prednisolone Acetate (10mg) per ml. (eyedrops)
Ketorolac Tromethamine 0.4%w/v + Moxifloxacin 0.5% w/v ophthalmicsolution
Loteprednol etabonate 0.5%w/v + Moxifloxacin 0.5% w/v ophthalmicSuspension
Moxifloxacin HCl BP 0.5%w/v + Bromfenac Sodium 0.09% w/v Eye drop
Moxifloxacin Eye Ointment(5mg/gm)
Cefixime (SR) 400mg +Moxifloxacin (SR) 400 mg Tablets
Note: Product protected by valid patents are not offered for sale incountries where such patents are still valid and its liability is at BuyersRisk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese