MO NTELUKAST
Product Details:
- Loss on Drying not more than 0.5 %
- HS Code 30049099
- Heavy Metal (%) not more than 10 ppm
- Solubility freely soluble in ethanol, methanol, and water, and practically insoluble in acetonitrile
- EINECS No 1308068-626-2
- Shelf Life 3 Years
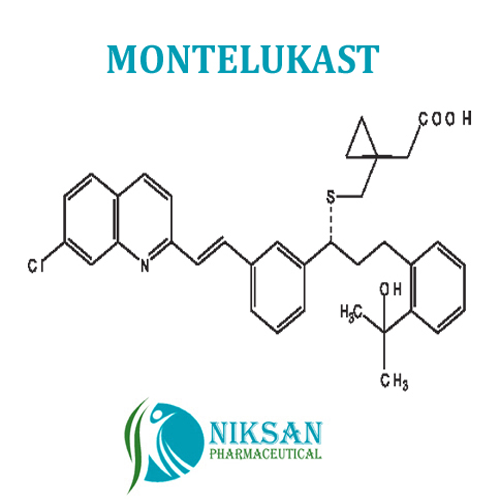
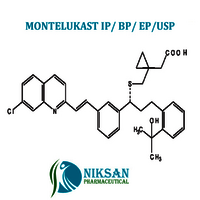
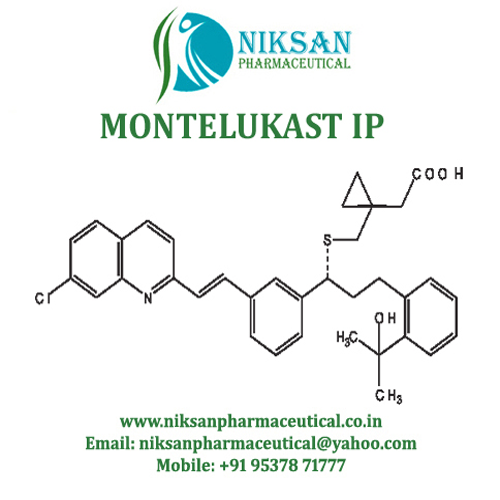
- Structural Formula C35H36ClNO3S
- Click to View more
MO NTELUKAST Price And Quantity
- 40000.00 INR/Kilograms
- 5 Kilograms
MO NTELUKAST Product Specifications
- 181 nm
- Other
- 750.5 C at 760 mmHg
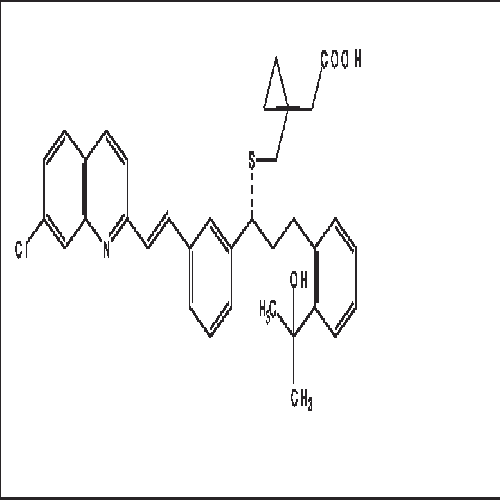
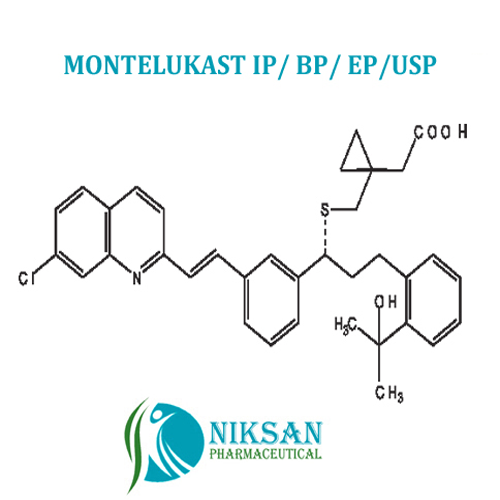
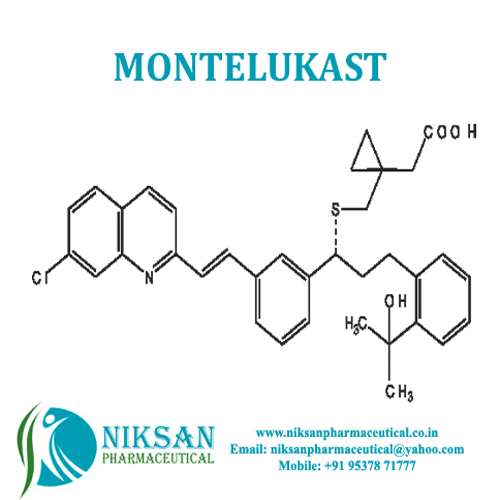
- [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt
- 9.610.0
- Room Temperature
- Take a one tablet of Sertraline in a one day. You can take Sertraline with or without food by mouth.Take everyday at any time for better improvement.
- C35H36ClNO3S
- Other
- white to off-white powder
- Powder
- 145-148C
- 30049099
- not more than 0.5 %
- freely soluble in ethanol, methanol, and water, and practically insoluble in acetonitrile
- 3 Years
- 1308068-626-2
- 99 %
- not more than 10 ppm
- Other
- MONTELUKAST
- C35H36ClNO3S
- Pharmaceutical Intermediates
- 586.18 g/mol Grams (g)
- 151767-02-1
MO NTELUKAST Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
is one of the biggest manufacturer, supplier, exporter and trader ofthe MONTELUKAST finished formulationsmoreover Montelukast API. Niksan Pharmaceutical is the huge manufacturer, exporter andsupplier of Montelukast API and formulation situated in Ankleshwar, Gujarat,India.NiksanPharmaceutical is the suppliers, manufacturer, exporters and trader of
Montelukast inthe domestic level as well as the internationalmarket.
Niksan is anIndian based pharmaceutical company so it also supplies and manufactures the Montelukast to allIndian states like Gujarat, Maharashtra, Punjab, Delhi, Tamilnadu, Goa, UttarPradesh, Karnataka, Jammu & Kashmir, West Bengal, Assam, Rajasthan, Hyderabad,Karnataka, Kerala, Madhya Pradesh, Manipur, Meghalaya, Mizoram, Nagaland,Orissa, Punjab and Haryana.
NiksanPharmaceutical and Niksan group companies are exporters, suppliers andmanufacturers of the API and also Montelukast formulations in many countries for many years. Niksanpharmaceutical currently exports Montelukast API and Formulation to countries like New Zealand, Jordan,Belgium, United Kingdom, Australia, Italy, Ireland, Hong Kong, Morocco,,Portugal, France, South Africa, Philippines, Malaysia, Singapore, Israel,Greece, Saudi Arabia, Egypt, Taiwan, Netherlands, South Korea, Switzerland,Algeria, Pakistan, Canada, Vietnam, United States, Germany, Brazil and manyother countries.
MONTELUKAST should be used totreat seasonal or perennial allergic rhinitis only in adults and children whocannot be treated with other medications. Montelukast is in a class ofmedications called leukotriene receptor antagonists (LTRAs).Montelukast is an orally dosed drug(available as a film-coated tablet, chewable tablet, or oral granules) that isFDA-approved for treating chronic asthma and prophylaxis and preventingexercise-induced bronchoconstriction.
SYNONYSMS:Montelukast sodium, Monteluk
IUPAC NAME: sodium2-[1-({[(1R)-1-[3-[(1E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2yl)phenyl]propyl]sulfanyl}methyl)cyclopropyl]acetate
CAS NO:158966-92-8
FORMULA:C35H36ClNO3S
MOLECULAR MASS: 586.187 g/mol
STORAGE CONDITIONS:Store the medicine in a closed containerat room temperature, away from heat, moisture, and direct light. Keep fromfreezing. Store the chewable tablets, oral granules, and tablets in itsoriginal package or container.
HOW TO USE:once a day in theevening to prevent symptoms of asthma or allergies. However, if exercise makesyour asthma worse, your doctor might tell you to take montelukast 2 hoursbefore you exercise. Never take more than 1 dose a day.
HOW MONTELUKASTWORKS:Montelukastis from a group of medicines called leukotriene receptor antagonists (LTRAs).LTRAs work by stopping the chemicals in your body that narrow your airways.These chemicals are called leukotrienes. Montelukast helps stop yourairways from narrowing (caused by inflammation).
PHARMACOKINETICSOF MONTELUKAST : At clinicallyrelevant concentrations, CYP2C8 appears to play a major role in the metabolismof montelukast. Elimination: Montelukast and metabolites are excreted almostsingly via the bile. The pharmacokinetics of montelukast is linear fordoses up to 50 mg.
SIDE EFFECTSOF MONTELUKAST : Common side effectsinclude headaches and stomach pain. Other potential side effects may includerespiratory infections, flu-like symptoms, digestive issues, rash, and earachesin children.Montelukast can cause serious mentalhealth changes. Monitor for any changes in mood, behavior, or thoughts andcontact your doctor immediately if they occur.
PRECAUTIONS: Inform your doctorabout any allergies to Montelukast, existing medical conditions like liverdisease or mental health issues, and if you are pregnant or breastfeeding.Chewable tablets contain aspartame and should be used cautiously in individualswith PKU. Discuss all medications, vitamins, and herbal products you are takingdue to potential interactions. Montelukast is not for acute asthma attacks;always have a rescue inhaler. Long-term use may require monitoring by yourdoctor.
CDSCOAPPROVAL:
Montelukast tabs 13/02/2002
Montelukast Sodium 10 mg Tablet, MontelukastSodium 4/5 mg Chewable Tablet and Montelukast Sodium Oral Granules (AdditionalIndication) 11.09.10
Montelukast Suspension 5mg/5ml 16.09.08
Montelukast Orally Disintegrating Strips4mg/5mg/10mg (additional Dosage form) 29.05.15
Montelukast sodium chewable tablet 4mg/5mgand Montelukast sodium film coated tablet 10mg. 26.02.02
Montelukast Granules (4mg/pack) 16/05/2005
Montelukast tablets 2004-September
Doxophylline (SR) 400mg/800mg + Montelukast10mg/10mg Tablets 07.04.10
Montelukast Sodium IP eq.to Montelukast 10mg+ Olopatadine Hcl 5mg Tablets 16.12.11
Fexofenadine 120mg + Montelukast 10mg31.08.10
Rupatadine 10 mg + Montelukast 10 mg Tablets10.11.09
Levocetirizine dihydrochloride 2.5mg +Montelukast 5mg dispersible tablet 13.10.10
Montelukast sod.+ bambuterol hcl tab10/04/2003
Fexofenadine 120mg + Montelukast 10mgChewable tablet 07.07.11
Theophylline 400mg SR + Montelukast 10mgTablet 31.03.09
FORMULATIONSAVAILABLE IN MARKET:
MONTELUKAST 4 MG tablets
MONTELUKAST 5 MG tablets
MONTELUKAST 10 MG tablets
MONTELUKAST SUSPENTION
Note: Productprotected by valid patents are not offered for sale in countries where suchpatents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese