ON DANSETRON
Product Details:
- Molecular Weight 293.4 g/mol Grams (g)
- Storage Room Temperature
- Smell Other
- Molecular Formula C18H19N3O
- EINECS No 103639-04-9
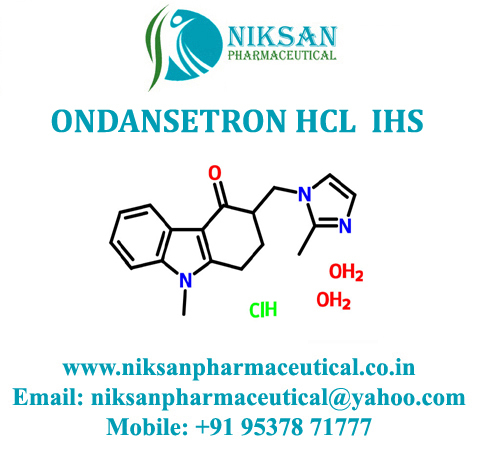
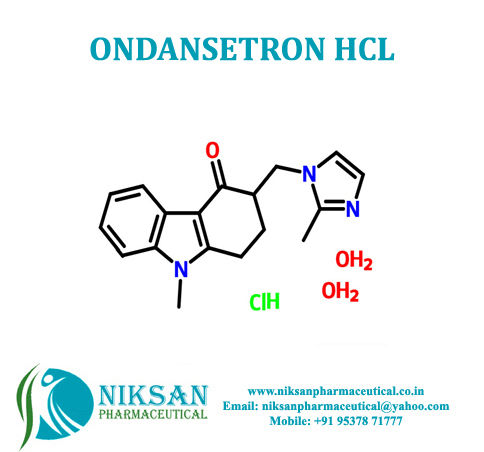
- Structural Formula C18H19N3O
- Boiling point 267-268 C
- Click to View more
ON DANSETRON Price And Quantity
- 5 Kilograms
ON DANSETRON Product Specifications
- White to Off-White Solid
- under the acidic conditions is water-soluble
- 267-268 C
- C18H19N3O
- 1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one
- Ondansetron used as anti-emetic agent. Ondansetron blocks the action of 5-HT3 receptor which causes the nausea and vomiting symptoms.
- 99614-02-5
- 103639-04-9
- C18H19N3O
- 99 %
- Other
- Room Temperature
- ONDANSETRON
- Other
- 293.4 g/mol Grams (g)
- 175.0 to 180.0 C
- between 1-5%
- 3.5 to 5.5
- Other
- Pharmaceutical Intermediates
- at least 80% are below 250 m
- 29420090
- 3 Years
- Powder
- NA
ON DANSETRON Trade Information
- NHAVA SHEVA
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
is one of the leading manufacturer, supplier and exporter of the Ondansetron. The best product manufactured by NiksanPharmaceutical is Ondansetron. We manufacture, supply andexport best quality product of OndansetronAPI along with Ondansetronformulations.
NiksanPharmaceutical is the largest manufacturer, trader and supply of Ondansetron in all-over Ankleshwar,Gujarat, India.
NiksanPharmaceutical manufacture and supply the Ondansetronproduct to the Indian states like Maharashtra, Goa,Sikkim, Madhya Pradesh, Uttar Pradesh, Assam, Haryana, Delhi, Bihar, Meghalaya,Tamilnadu, Kerala, Gujarat, Rajasthan, Rajasthan, Punjab,Karnataka, and otherstates.
is manufacturer , supplier and exporter of Ondansetronin many countries from many years like New Zealand, United States,Paraguay, Indonesia, Mexico, Australia, Chile, Nepal, Venezuela, Ecuador,Puerto Rico, Colombia, Uruguay, Kenya, Bolivia, Canada, Peru, Bangladesh,Portugal, Ireland, United Kingdom, Iraq, United Arab Emirates, Singapore,Sweden, Iran, Pakistan, Spain, Argentina, Philippines, Saudi Arabia, Finland,Denmark, Switzerland, Netherlands, Germany, South Africa, Thailand, Egypt,Austria, Malaysia, Italy, Belgium, France, Poland, Vietnam, Turkey, Brazil andmany more countries.
Ondansetron belongs to5-HT3 antagonist class of medicine. Ondansetron used as an anti-emetic agent toprevent nausea and vomiting caused by the cancer therapy. It is also used toprevent vomiting caused by motion sickness.
IUPAC NAME: 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-2, 3, 4, 9-tetrahydro-1H-carbazol-4-one
CAS NO: 99614-02-5
FORMULA: C18H19N3O
MOLECULAR MASS: 293.4 g/mol
STORAGECONDITIONS: Store in cool and dry place, away from heat and light. Do not put inbathroom or ant misty place. Keep away from children.
HOW TO USE: This medication come inmouth dissolving formulation, so do not chew or break the medicine. Placemedicine on the tip of the tongue and allow dissolving it. Swallow the medicineafter dissolves in the mouth.
HOW ONDANSETRONWORKS: Ondansetronused as anti-emetic agent. Ondansetron blocks the action of 5-HT3 receptorwhich causes the nausea and vomiting symptoms.
PHARMACOKINETICS: Ondansetron absorbed in GI after the oral administration.Almost 73% drug binds with the plasma protein. The half life of Ondansetron is3-4 hours. Ondansetron eliminated from body by urination and feces.
SIDE EFFECTS: Side effects cause by Ondansetron isdizziness, light headedness, drowsiness, headache, tiredness. Other side effects like chest pain, irregularheartbeats, fainting, and abdominal pain. Muscle pain seen in the patients.
PRECAUTIONS: Before taking themedication tell your doctor if you are allergic towards the medicine. The medicinemakes you dizzy so kindly avoid drinking alcohol and cannabis. This drug makesyou dizzy so kindly avoid driving or operating machineries.
CDSCO APPROVAL: R-Ondansetron (As HCl. Dihydrate) (1mg/ml)injection approved by CDSCO in India in 12.01.2007,
Ondansetron Fast Dissolving Strips 4mg/8mg (New dosage form) approvedby CDSCO in India in22.11.2011
Ondansetron orally disintegrating tablet (4mg/8mg) (additionaldosage form)approved by CDSCO in India in 10.10.2007
Ondansetron tablet and injection approved by CDSCO in India in12.02.1994
Ondansetron Rapid Film Oral Strip 4/8 mg approved by CDSCO inIndia in16.11.2010
Ondansetron Hydrochloride Mouth Melting Strips 4mg approved byCDSCO in India in29.03.2012
R (+) Ondansetron (2mg/4mg) tablet approved by CDSCO in India in15.04.2005
Ondansetron Hydrochloride Oral Spray 2mg (Each spray deliversondansetron-2mg)approved by CDSCO in India in 08.11.2012
R (-) Ondansetron oral Solution (1mg/5ml) approved by CDSCO inIndia in27.05.2005
FORMULATIONS AVAILABLE IN MARKET:
Ondansetron Fast Dissolving Strips 4mg
Ondansetron Fast Dissolving Strips 8mg
Ondansetron 4mg orally disintegrating tablets
Ondansetron 8mgorally disintegrating tablets
Ondansetron injections
Ondansetron Rapid Film Oral Strips 4mg
Ondansetron Rapid Film Oral Strips 8mg
R (+) Ondansetron 2mg tablets
R (+) Ondansetron 4mg tablets
R (-) Ondansetron 1mg/5ml oral Solution
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
How Ondansetron Works
Ondansetron functions as a 5-HT3 receptor antagonist, interrupting the signals that trigger nausea and vomiting. By acting on these receptors, particularly in the gastrointestinal tract and brain, it is highly effective for patients undergoing cancer therapy or surgery.
Dosage and Administration Guidance
The mouth dissolving formulation should not be chewed or broken. Instead, place the tablet on your tongue and allow it to dissolve completely before swallowing. Always follow your healthcare providers instructions for dosage, and do not exceed recommended amounts.
Key Storage Instructions
For optimal stability and effectiveness, store Ondansetron in a cool, dry environment, shielded from direct sunlight and heat. Ensure the container is tightly closed to preserve the products integrity over its 3-year shelf life.
FAQs of ONDANSETRON:
Q: How should I take Ondansetron mouth dissolving tablets?
A: To use the mouth dissolving tablet, place it on the tip of your tongue and allow it to dissolve fully. Do not chew or break the tablet. Swallow after it completely dissolves in your mouth.Q: What is Ondansetron commonly used for?
A: Ondansetron is primarily used to prevent and manage nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgical procedures.Q: When should Ondansetron be administered for best results?
A: Following your doctors instructions is essential. Typically, Ondansetron should be given before the start of chemotherapy, radiation, or surgery, and may be continued as recommended to prevent or manage symptoms.Q: Where should Ondansetron powder be stored to maintain its quality?
A: The powder should be stored at room temperature in a cool, dry place, away from direct sunlight and heat. Always keep the container tightly sealed.Q: What are the benefits of using Ondansetron?
A: Ondansetron provides effective relief from nausea and vomiting, improving comfort and quality of life for individuals undergoing treatments known to cause these side effects.Q: Is Ondansetron available in forms other than tablets?
A: Yes, Ondansetron is also available in injectable and oral solution forms, allowing flexibility in administration based on patient needs.Q: Can Ondansetron be used for other gastric issues?
A: While primarily indicated for nausea and vomiting from chemotherapy, radiation, or surgery, Ondansetron may sometimes be used as prescribed by a healthcare professional for similar gastric complaints.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese