BO SENTAN
Product Details:
- Boiling point 742.3 C
- Structural Formula C27H29N5O6S
- Smell Other
- HS Code 293500
- EINECS No not available
- Melting Point 200-202C
- Ph Level pH 1.1, 4.0, and 5.0
- Click to View more
BO SENTAN Price And Quantity
- 1 Kilograms
- 140000 INR/Kilograms
BO SENTAN Product Specifications
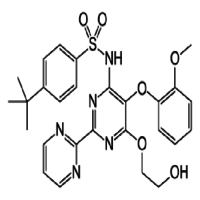
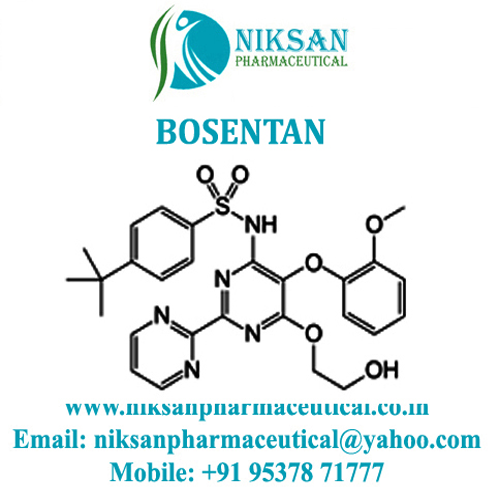
- 4-(tert-Butyl)-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)[2,2-bipyrimidin]-4-yl]benzenesulfonamide, monohydrate
- Other
- 742.3 C
- 147536-97-8
- Other
- C27H29N5O6S
- 293500
- not available
- 200-202C
- 99%
- pH 1.1, 4.0, and 5.0
- Other
- not known to contain
- C27H29N5O6S
- Bosentan IS treat the symptoms of pulmonary arterial hypertension (PAH) in adults or in children 3 years of age or older
- Room Temperature
- 200 nm for nanoparticles to 1-5 m
- 551.614 g/mol Grams (g)
- 55 and 75C
- less than 2 g/mL in water
- BOSENTAN
- Powder
- 3 Years
- white to off-white or pale yellow
- Other
BO SENTAN Trade Information
- INDIA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Day
- 1 Days
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- GMP, FDCA
Product Description
is the largest manufacturer and exporter of the Bosentan API among all over the world. Niksan Pharmaceutical is oneof biggest the supplier, manufacturer, exporter and distributor of the Bosentan API and finished formulationsin Ankleshwar, Gujarat, India. The products of Niksan Pharmaceutical andNiksan group companies are widely appreciated by the clients and othercompanies.
Niksan Pharmaceutical provides API and finishedformulations of Bosentan in all overIndian states Like Kerala, Gujarat, Haryana, Rajasthan, Madhya Pradesh, UttarPradesh, Rajasthan, Karnataka, Meghalaya, Tamilnadu, Goa, Sikkim, Assam,Punjab, Delhi, Bihar, Jammu Kashmir Etc.
Niksan pharmaceutical is also large exporter ofthe API and finished pharmaceutical products of Bosentan in many countries for years. The countries where weexporting are Colombia, Iraq, Paraguay, Switzerland, Argentina, Mexico,Pakistan, South Korea, Italy, Germany, Romania, Saudi Arabia, Philippines, Egypt,Spain, Portugal, Australia, United States, Tunisia , Canada, Iran, Turkey,Chile, United Kingdom, Taiwan, Austria, Netherlands, France, Belgium, Peru,Thailand, Venezuela, Vietnam, Poland, Brazil, Indonesia and many morecountries.
Bosentan is the medicine used inthe treatment of pulmonary artery hypertension.
Bosentan belongs to endothelin receptor antagonist. Bosentanincrease blood flow in patient suffering from PAH.
SYNONYMS OF BOSENTAN: Bosentn, Bosentan, Bosentanum.
IUPAC NAME: 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2(pyrimidin-2-yl)pyrimidin-4-yl] benzene-1-sulfonamide
CAS NO: 147536-97-8
FORMULA: C27H29N5O6S
MOLECULAR MASS: 551.61 gmol 1
STORAGE CONDITIONS: Store in cool and dry place, keep away fromdirect heat and light. Do not put in humid place like bathroom. Keep away fromchildren and pet.
HOW TO USE: Take this medicine 2 times per day, normallyin morning and evening.
Take medicines by mouth with or without water. Put you tablet insmall amount of water and dissolve it, when it dissolves drink whole mixture atonce.
HOW BOSENTANWORKS:
PHARMACOKINETICS: Bosentanhave very low solubility so Bosentan absorbed when it taken in high amount. Thedrug binds with almost 98% of plasma protein. Bioavaibility of this medicine is505 and it is not affected by food. Half-life of Bosentan in health person is 5hrs. The Bosentan excited by the biliaryexcretion.
SIDE EFFECTSOF BOSENTAN: Runny nose, dizziness, flushing and joint pain are the common sideeffects seen in the patients. If you see symptoms like swelling of ankles,fainting, weight gain, shortness of breath, fast heartbeat, weakness tell yourdoctor about this problems. This drug can effects on sperm production and alsoaffect your ability to become father. The allergic reaction of Bosentan israre.
PRECAUTION: Tell your doctor if youhave allergic reaction towards the Bosentan.
Talk with your doctor if you have other disease like liver orkidney problems. This rug cause dizziness so kindly avoid driving and heavyexercise. Consumption of alcohol or cannabis can make you dizzier so kindlyavoid it while taking the medicament. If you must not be taken in pregnancybecause it can harms the unborn baby.
CDSCO APPROVAL: Bosentan (as monohydrate) Tablets62.5mg/125mg approved by CDSCO in Indian in 23.06.2009.
FORMULATIONS AVAAILABLE INMARKET:
Bosentan 62.5mg tablets
Bosentan 125mg tablets
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese