LUMEFANTRINE
Product Details:
- Melting Point 128C to 138C
- HS Code 29420090
- Storage Keep away from moisture
- Particle Size 100 nm or less
- EINECS No 617-303-4
- Molecular Formula C30H32Cl3NO
- Smell Other
- Click to View more
LUMEFANTRINE Price And Quantity
- 5 Kilograms
LUMEFANTRINE Product Specifications
- Other
- 128C to 138C
- 29420090
- Keep away from moisture
- Pharmaceutical Intermediates
- LUMEFANTRINE
- C30H32Cl3NO
- 82186-77-4
- C30H32Cl3NO
- Take medication 2 times per day with meal for three day. Take your doctors advice before taking the medication. If you vomit after taking the drug, take medicine again after 30 minutes.
- Other
- 100 nm or less
- 617-303-4
- Powder
- water is less than 50 ng/mL
- 760 mmHg
- less than 2%
- 1.2
- Other
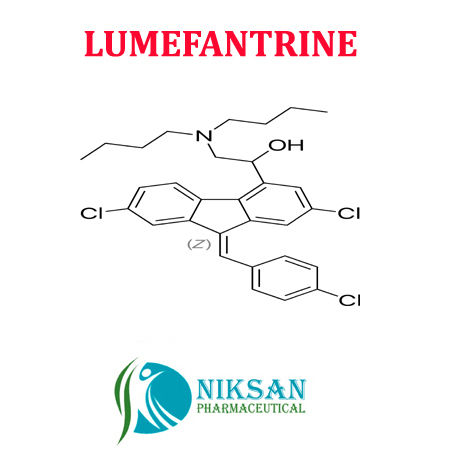
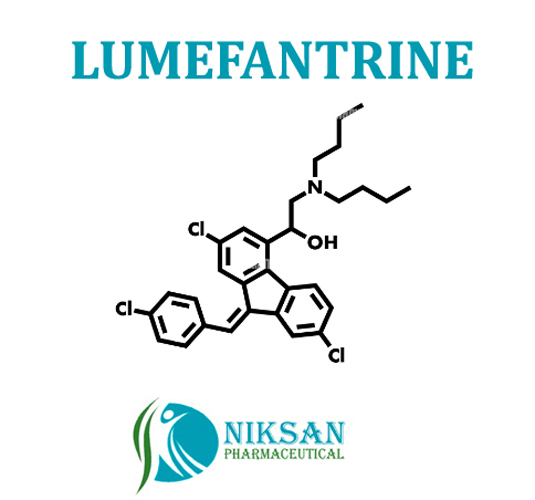
- (9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]--[(dibutylamino)methyl]-9H-fluorene-4-methanol
- 3 Years
- 99 %
- White to Off-White Solid
- 528.94 g/mol Grams (g)
LUMEFANTRINE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical is one of the leading manufacturer,supplier and exporter of the Lumefantrine.The best product manufactured by Niksan Pharmaceutical is Lumefantrine. We manufacture, supply and exportbest quality product of Lumefantrine API along with Lumefantrine formulations.
Niksan Pharmaceutical is also manufacture and exports largequantity of Lumefantrine products in whole globe since many years incountries like Nepal, United Arab Emirates, South Korea, Philippines,Bangladesh, Australia, United States, United Kingdom, Bulgaria, Georgia,Germany, Ghana, Greece, Grenada, Guatemala, Guinea, Guinea-Bissau, Guyana,Haiti, Honduras, Hungary, Iceland, , Indonesia, Iran, Iraq, Ireland, Israel,Italy, Jamaica, Colombia, Comoros, Democratic Republic of the Congo, Republicof the Congo, Costa Rica, Cote dIvoire, Croatia, Cuba, Cyprus, Czech Republic,Denmark, Djibouti, Dominica, Dominican Republic, East Timor (Timor-Leste),Ecuador, Egypt, El Salvador, Equatorial Guinea, Eritrea, Japan and Jordan, Kazakhstan,Kenya, Kiribati, North Korea, South Korea, Kosovo, Kuwait, Kyrgyzstan, LaosBurkina Faso, Burundi, Estonia, Eswatini, Ethiopia, Mauritius, Mexico, Lebanon,Lesotho, Liberia, Libya, Liechtenstein, Maldives, Mali, Malta, MarshallIslands, Mauritania, Peru, Philippines,Poland, Micronesia, Moldova, Monaco,Mongolia, Montenegro, Morocco, Mozambique, Myanmar (Burma), Namibia, Nauru,Nepal, Netherlands, New Zealand, Nicaragua, Niger, Nigeria, North Macedonia,Norway, Oman, Pakistan, Palau, Panama, Papua New Guinea, Paraguay, Fiji,Finland, France, Gabon, The Gambia, , Latvia, Portugal, Qatar, Romania, Russia,Rwanda, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines,Cabot Verde, Cambodia, Cameroon, Canada, Central African Republic, Chad, Chile,China, Samoa, San Marino, Sao Tome and Principe and Saudi Arabia and many othercountries.
Niksan Pharmaceutical provides best quality product of Lumefantrine in all states of Indialike Kerala,Punjab, Rajasthan, Delhi, Tamil Nadu, Odisha, Madhya Pradesh,Telangana, Bihar,Karnataka, Jammu and Kashmir, Maharashtra, West Bengal, Uttar Pradesh,Gujarat, Andhra Pradesh, Haryana and many other states.
Lumefantrine is the antimalarial drug. Lumefantrineused in the treatment of malaria caused by the mosquito bite. Lumefantrine ismember of monochlorobenzenes. It is secondary alcohol. Lumefantrine belongs tothe medicine class called fluorenes.
SYNONYMS: Benflumetol, dl-Benflumelol, Lumefantrine
IUPAC NAME: 2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl) methylidene]-9H-fluoren-4-yl]ethan-1-ol
CAS NO: 82186-77-4
FORMULA: C30H32Cl3NO
MOLECULAR MASS: 528.94 g/mol
STORAGE CONDITIONS: Store in roomtemperature in dry place. Do not keep medication in humid place or bathroom. Keepmedication away from direct light and heat. Keep away from children and pets.
HOW TO USE: Take medication 2times per day with meal for three day. Take your doctors advice before takingthe medication. If you vomit after taking the drug, take medicine again after30 minutes.
HOW LUMEFANTRINEWORKS: Lumefantrine kills the plasmodium parasites by inhibiting the nucleicacid synthesis inside them. And by this process Lumefantrine gives antimalarialeffect to the patient.
PHARMACOKINETICS:Lumefantrine have good absorption rate inthe body, it absorbs more when taken with meal. Lumefantrine bounds with almost99.7% of plasma proteins. The half-life of Lumefantrine is 4.5days. TheLumefantrine metabolizes by the liver and after that it is eliminated throughthe bile. The elimination half-life of Lumefantrine is 3-6 days.
SIDE EFFECTS: The side effects of Lumefantrine arefever, chills, muscle pain, nausea, vomiting, headache, abdominal pain,insomnia, cough, dizziness and many more. Contact your doctor if you see sideeffects like fainting, chest pain, and dizziness, fast or irregular heartbeat. Theallergic reaction of this drug is rare but if you see effects like rash,itching, or swelling of skin.
PRECAUTIONS: Lumefantrine cause heart problems soconsult your doctor before taking the medication. Tell your doctor if you areallergic to Lumefantrine or inactive ingredients. Take medical attention rightaway if you see any effects like dizziness, fainting, fast irregular heartbeatsor any heart problem. If you have conditions such as sweating, vomiting, motionsickness tell your doctor about it.
CDSCO APPROVAL: Artemether + Lumefantrine approved by CDSCO in India in 26.12.2002,
Artemether 40mg/80mg + Lumefantrine 240mg/480mg tablets(additional Strength) approved by CDSCO in India in 24.06.2008,
Artemether + LumefantrineDispersible tablet (40/80mg + 240/480mg) (Additional Strength) approved byCDSCO in India in 05.08.2008,
Artemether + Lumefantrine (80mg + 480mg/5ml) Dry syrupapproved byCDSCO in India in 30.09.2008
FORMULATIONS AVAILABLE IN MARKET:
Artemether 80mg + Lumefantrine 480mg tablets
Artemether 20mg + Lumefantrine 120mg/ml oral solution
Artemether40mg + Lumefantrine240mg tablets
Artemether 20mg + Lumefantrine 120mg tablets
Artemether 40mg + Lumefantrine 240mg/5ml oral solution
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
How to Take LUMEFANTRINE Effectively
For optimal anti-malarial results, LUMEFANTRINE should be administered twice daily with meals over a three-day period. Always consult your physician before use, especially if you have pre-existing health conditions or require specific dosage adjustments. In the event of vomiting shortly after ingestion, repeat the dose after 30 minutes.
Safe Storage and Handling Instructions
Store LUMEFANTRINE in its original container at room temperature, away from moisture and humidity. Avoid placing the medication in bathrooms or other damp areas, as moisture can affect its stability and efficacy. Proper storage helps maintain its effectiveness throughout its three-year shelf life.
Benefits and Recommended Usage
Lumefantrine is designed specifically for treating malaria resulting from mosquito bites. Its high purity and stability make it a reliable option for those in malaria-prone areas. Taking the medication as prescribed helps ensure effective clearance of the malarial infection, with minimal risk of recurrence.
FAQs of LUMEFANTRINE:
Q: How should I take LUMEFANTRINE for best results?
A: Take LUMEFANTRINE twice daily with meals for three consecutive days. Follow your doctors advice concerning dosage and duration; if you vomit within 30 minutes of a dose, repeat the dosage accordingly.Q: What is the primary benefit of using LUMEFANTRINE?
A: LUMEFANTRINE is highly effective in treating malaria caused by mosquito bites. Its targeted action helps eradicate malarial parasites and reduces the risk of recurrent infection.Q: Where should I store LUMEFANTRINE powder to maintain its quality?
A: Keep LUMEFANTRINE stored in a dry place at room temperature. Do not store it in humid areas such as bathrooms, as moisture can compromise its potency.Q: What should I do if I vomit after taking LUMEFANTRINE?
A: If you vomit within 30 minutes after taking the medication, take the same dose again to ensure you receive the full therapeutic benefit.Q: When is LUMEFANTRINE recommended for use?
A: LUMEFANTRINE is recommended for adults and children as part of combination therapy for treating malaria, following a diagnosis and doctors prescription.Q: Is LUMEFANTRINE safe to use for all individuals?
A: Consult your doctor prior to taking LUMEFANTRINE, especially if you have underlying health conditions or are taking other medications, to ensure safety and appropriateness.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese