LE VETIRACETAM
Product Details:
- Loss on Drying less than 0.5%
- Taste Other
- EINECS No 600-348-9
- Solubility in water (104.0 g/100 mL)
- Boiling point 395.9 C
- Molecular Formula C8H14N2O2
- Storage Keep away from moisture
- Click to View more
LE VETIRACETAM Price And Quantity
- 8 Kilograms
- 3200.00 INR/Kilograms
LE VETIRACETAM Product Specifications
- LEVETIRACETAM
- Levetiracetam is one type of antiepileptic agent which is used to prevent partial seizure in adults and also used in children. Levetiracetam come in the category of anticonvulsant class of drug which is used in seizures. Levetiracetam prevents seizure by inhibiting the excitement level of brain. Levetiracetam used to prevent many type of seizure like partial seizures, myoclonic seizures, epilepsies etc.
- 116.0 to 120.0 C
- Other
- 170.21 g/mol Grams (g)
- C8H14N2O2
- Other
- C8H14N2O2
- Other
- LEVETIRACETAM
- Keep away from moisture
- 3 Years
- 29420090
- Powder
- pH 5.5
- 120-200 m
- 600-348-9
- 99 %
- in water (104.0 g/100 mL)
- 395.9 C
- 102767-28-2
- less than 0.5%
- white to off-white crystalline powder
- Other
LE VETIRACETAM Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical is one of the leading manufacturer,supplier and exporter of the antiepileptic medication. The best antiepilepticproduct manufactured by Niksan Pharmaceutical is Levetiracetam. We manufacture, supply and export best quality productof Levetiracetam API along with Levetiracetam formulations.
Niksan Pharmaceutical provides best quality product of Levetiracetam in all states of Indialike Kerala,Punjab, Rajasthan, Telangana, Bihar, Karnataka, Jammu and Kashmir, Maharashtra,West Bengal, Uttar Pradesh, Gujarat, Delhi, Tamil Nadu, Odisha, Madhya Pradesh,Andhra Pradesh, Haryana and many otherstates.
Niksan Pharmaceutical is also manufacture and export largequantity of Levetiracetam productsin whole globe since many years in countries like Chile, Mexico, Colombia, andPuerto Rico, Portugal, Germany, New Zealand, Dominican Republic, United States,Argentina, Austria, Panama, Romania, Ecuador, Guatemala, Uruguay, Paraguay, UnitedKingdom, Philippines, Spain, Canada, Jordan, Ireland, Australia, Netherlands,United Arab Emirates, Italy, Pakistan, Slovakia, Switzerland, Sweden,Venezuela, Singapore, Iraq, Hungary, Peru, Bolivia, Saudi Arabia, Denmark,Poland, France, Malaysia, Taiwan, South Korea, Israel, Finland, Czechia, Egypt,Hong Kong, Belgium, Thailand, Brazil, Greece, Iran, South Africa, Vietnam,Indonesia, Turkey, Japan and many other countries.
Levetiracetam is one type of antiepileptic agent whichis used to prevent partial seizure in adults and also used in children.
Levetiracetam come in the category of anticonvulsantclass of drug which is used in seizures.
Levetiracetam prevents seizure by inhibiting theexcitement level of brain. Levetiracetam used to prevent many type of seizurelike partial seizures, myoclonic seizures, epilepsies etc.
SYNONYMS OFLEVETIRACETAM: levetiracetam, levetiracetame, levetiracetamum.
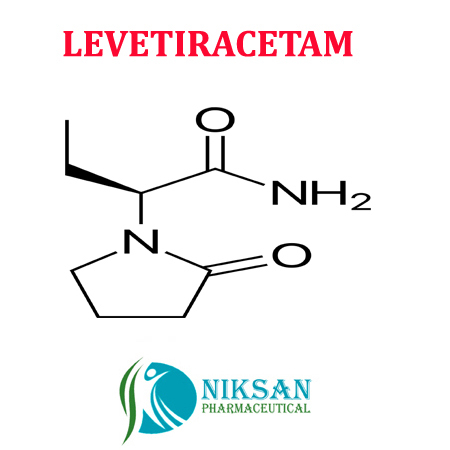
IUPACNAME: (2S)-2-(2-oxopyrrolidin-1-yl)butanamide
CASNO: 102767-28-2
MOLECULARFORMULA: C8H14N2O2
MOLECULAR MASS: 170.21 g/mol
STORAGE CONDITIONS OF LEVETIRACETAM: Store theLevetiracetam medication in a cool and dry place away from the direct sunlightor flames. Do not put medication in kitchen or bathroom. Keep medication in itsoriginal container. Keep medicines away from reach of pets and children.
HOW TO USE: Levetiracetam comes in tablet form. Takemedications advised by your doctor, take medicine 2 times per day with orwithout food. Take medication regularly for the better result, dont miss thedose and if missed the dose take it when you remember.
PHARMACOKINEICS OF LEVETIRACETAM: Levetiracetam havebioavailability of 100% in the body. Levetiracetam rapidly and completelyabsorbed with the oral administration. The time taken by Levetiracetam to reachthe peak plasma concentration is 1-1.5 hrs. The approx. half-life ofLevetiracetam is 6-8 hrs. Normally 66% of Levetiracetam and residues willexcrete by urination.
SIDE EFFECTS OF LEVETIRACETAM: The common sideeffects of Levetiracetam are dizziness, weakness, tiredness, drowsiness,headache are seen in the patient. Tell your doctor immediately if you findsymptoms like mood change, loos of coordination, suicidal thoughts, fever,chills, our throat, unusual behaviour. There are some rare allergic reactionsalso caused by Levetiracetam in patient like rash, itching, swelling ofthroat/tongue etc.
PRECAUTIONS OF LEVETIRACETAM: Tell your doctor ifyou have diagnosed with any kidney, heart problem or any mental disorders likedepression. The drug will make you dizzy and because blurred vision so do nottry to drive car or operate vehicles. Dont do any exercise or heavy lifting. Thedrug cause dizziness so does not take any alcoholic product or cannabis. Alwaystake medication on time, on a regular interval.
CDSCO APPROVAL: Levetiracetam SR Tablet 500/750/1000mg tablets approved by CDSCOin India in 20.05.2009,
Levetiracetam Tablet (addl. indication) approved by CDSCO in Indiain 09.10.2007,
Levetiracetam (1000mg) tablets approved by CDSCO in Indian in may2005, Levetiracetam Oral Solution (100mg/ml) approved by CDSCO in India in24.02.2006,
Levetiracetam (250mg, 500mg, and 750 mg) tablets approved by CDSCOin India in 05/04/2005, Levetiracetam Oral Solution 100mg/ml (AdditionalIndication) approved CDSCO in Indian in 05.05.2011,
Levetiracetam Oral Solution(100mg/ml) approved by CDSCO in India in 04.10.2005.
FORMULATIONS AVAILABLE IN MARKET:
Levetiracetam 250mg tablets
Levetiracetam 500mgtablets
Levetiracetam 750mgtablets
Levetiracetam SR500mg tablets
Levetiracetam SR750mg tablets
Levetiracetam SR1000mg tablets
Levetiracetam Tablet (addl. indication)
Levetiracetam Oral Solution (100mg/ml)
Levetiracetam Oral solution (500mg/ml)
Levetiracetam 500mg +NACL 750 mg/ml oral solution
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese