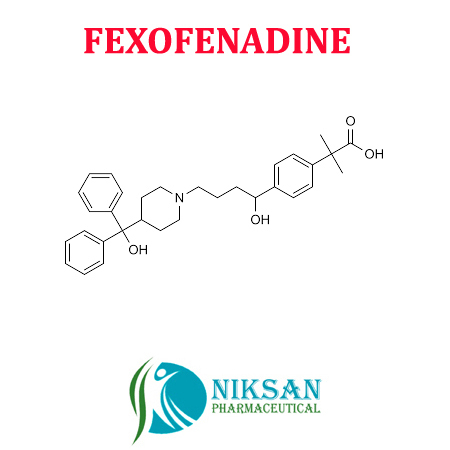
FEXOFENADINE
Product Details:
- Storage Room Temperature
- Heavy Metal (%) less than 20ppm
- Shelf Life 3 Years
- EINECS No 604-906-2
- Smell Other
- Melting Point 190-192C
- Particle Size 10 to 100 micrometers
- Click to View more
FEXOFENADINE Price And Quantity
- 5 Kilograms
- 4500 INR/Kilograms
FEXOFENADINE Product Specifications
- 697.3 C
- 10 to 100 micrometers
- 99 %
- less than 1%
- 3.0 to 4.5
- 501.68 g/mol Grams (g)
- Fexofenadine has a solubility of 3.6 mg/mL in water
- FEXOFENADINE
- Other
- 29420090
- Other
- C32H39NO4
- C32H39NO4
- Medicine Grade
- Room Temperature
- Powder
- less than 20ppm
- ()-4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]-,-dimethyl benzeneacetic acid hydrochloride
- 83799-24-0
- 3 Years
- Fexofenadine belongs to the drug class called antihistamines. Fexofenadine used in the allergic treatment like watery eye, swollen skin, rash, itching or irritation. Fexofenadine prevents the allergic reaction by blocking the effects of histamine.
- 604-906-2
- Other
- 190-192C
- White to Off-White Solid
FEXOFENADINE Trade Information
- INDIA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Dadra and Nagar Haveli, Chandigarh, Himachal Pradesh, Andaman and Nicobar Islands, Uttarakhand, Daman and Diu, Lakshadweep, Nagaland, South India, Central India, North India, East India, West India, Andhra Pradesh, Assam, Arunachal Pradesh, Bihar, Delhi, Gujarat, Goa, Haryana, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Mizoram, Meghalaya, Manipur, Odisha, Punjab, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Uttar Pradesh, West Bengal, Chhattisgarh, All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical is worlds leading manufacturer, trader, exporter and supplier of the Fexofenadine API and Fexofenadineformulations. Niksan Pharmaceutical manufactures large quantity of FexofenadineAPI and finished products in Ankleshwar, Gujarat, India. Niksan Pharmaceuticaland Niksan group companies are the largest manufacturers and supplier ofFexofenadine products.
Niksan Pharmaceutical exporting very big quantity of the fines quality products of Fexofenadine in all over world for manyyears in a countries like Maldives,Nepal, Bangladesh, Sri Lanka, United Kingdom, Puerto Rico, United States,Australia, Vietnam, Singapore, Qatar, Pakistan, United Arab Emirates, HongKong, Taiwan, Netherlands, Thailand, Kenya, Malaysia, Jordan, Ireland, New Zealand,South Africa, Iraq, Saudi Arabia, Israel, Nigeria,Philippines, Iran,Egypt, Indonesia, Switzerland, Canada, South Korea, France,Belgium, Colombia, Germany, Mexico, Poland, Japan, Spain, Brazil, Turkey, Italyand many more countries.
Niksan Pharmaceutical provides API and finishedformulations of Fexofenadine in all over Indian states Like JammuKashmir, Kerala, Gujarat, Haryana,Rajasthan, Madhya Pradesh, Uttar Pradesh, Rajasthan, Karnataka, Meghalaya,Tamilnadu, Goa, Sikkim, Assam, Punjab, Delhi, Bihar Etc.
Fexofenadine belongs to the drug class called antihistamines. Fexofenadine used inthe allergic treatment like watery eye, swollen skin, rash, itching orirritation. Fexofenadine prevents the allergic reaction by blocking the effectsof histamine.
SYNONYMS OF FEXOFENADINE: Carboxyterfenadine, Fexofenadina,Fexofenadine, Terfenadine acid metabolite, Terfenadine carboxylate,Terfenadine-COOH.
IUPAC NAME:2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butyl}phenyl)-2-methylpropanoic acid
CAS NO: 83799-24-0
FORMULA: C32H39NO4
MOLECULAR MASS: 501.68 g/molgmol 1
STORAGE CONDITIONS: Store in cool anddry place, away from the direct heat and light. Do not put Fexofenadinemedication in bathroom and humid place. Keep away from children and pets.
HOW TO USE: Take medication directly by mouth 2 timesper day after 12 hours. If you use oral solution, kindly shake well before use.Take your doctors advice before using the medication.
HOW FEXOFENADINE WORKS: Fexofenadine blocks the effects ofhistamine which cause allergic reaction. By blocking the histamine effectsFexofenadine prevents the allergic reactions like skin rash, watery eyes,itching and irritation which is caused by the other medication.
PHARMACOKINETICS OF FEXOFENADINE: Fexofenadine absorbsrapidly after the oral administration. Fexofenadine take 1-3 hours to reach thepeak plasma concentration. Almost 60-70% of Fexofenadine binds with the bloodplasma protein. The half-life of Fexofenadine is between 11-15 hours. Almost80% of Fexofenadine eliminated by feces and only 11% residues eliminatedthrough urination.
SIDE EFFECTS OF FEXOFENADINE: The common sideeffects like ever, cold and stomach upset seen some time. Some rare sideeffects like allergic reactions like rash, itching, swelling of face, tongue, dizzinessand trouble in breathing are caused by Fexofenadine medication.
PRECAUTION: Kindly tell your doctor if you havediabetes, PKU and other blood sugar related problem. Tell your doctor if youare allergic to the Fexofenadine medication.
Tell your doctor ifare already taking other medication. If you have kidney disease or liverdisease kindly tell your doctor.
CDSCO APPROVAL: Fexofenadine 120mg + Montelukast 10mg tablet approved by CDSCO inIndia in 31.08.2010,
Fexofenadine HCl tablets approved by CDSCO in India in 16.09.1998,
Fexofenadine suspension (30mg/5ml) approved by CDSCO in India in19.01.2007,
Fexofenadine + pseudo ephedrine ER Tabs approved by CDSCO in Indiain 17.09.1999,
Fexofenadine HCl approved by CDSCO in India in 01.11.2000,
Fexofenadine HCl suspension approved by CDSCO in India in27.08.2001,
Fexofenadine 120mg + Montelukast 10mg Chewable tablet approved by CDSCOin India in 07.07.2011
FORMULATION AVAILABLE IN MARKET:
Fexofenadine 120mg + Montelukast 10mg tablets
Fexofenadine HCl tablets
Fexofenadine suspension 30mg/5ml
Fexofenadine + pseudo ephedrine ER Tablets
Fexofenadine HCl suspension
Fexofenadine 120mg + Montelukast 10mg Chewable tablets
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
How Fexofenadine Works
Fexofenadine is classified as an antihistamine, meaning it inhibits the action of histamine, a chemical responsible for allergy symptoms. By blocking the histamine receptors, Fexofenadine helps alleviate common allergic responses, such as skin swelling, watery eyes, rashes, and itching, providing effective relief for patients.
Usage and Recommended Dosage
For managing allergies, Fexofenadine is most often taken orally as tablets or suspension. Adults and children are usually advised to take the medication twice a day, about 12 hours apart. When using the oral solution, it should be shaken well before use for optimal effectiveness. Always follow prescribed dosages for safe use.
Proper Storage Instructions
Fexofenadine should be stored at room temperature in a cool, dry environment, away from direct sunlight and heat sources. Avoid places with humidity, such as the bathroom, to prevent degradation of the medications quality. Proper storage ensures the product remains effective throughout its three-year shelf life.
FAQs of FEXOFENADINE:
Q: How does Fexofenadine help treat allergic symptoms?
A: Fexofenadine works by blocking the effects of histamine, a substance in the body that causes allergy symptoms like watery eyes, itching, swollen skin, and rashes. By preventing histamine from attaching to its receptors, Fexofenadine helps alleviate and control these symptoms effectively.Q: What are the recommended instructions for storing Fexofenadine?
A: Fexofenadine should be kept in a cool, dry location away from direct heat and light. Do not store it in humid areas such as bathrooms, as moisture and temperature changes can affect its stability and effectiveness.Q: When should I take Fexofenadine for best results?
A: Fexofenadine is typically taken twice a day, about 12 hours apart. For oral suspension, always shake the bottle well before using. Taking the medication at consistent times daily ensures steady relief from allergy symptoms.Q: What forms of Fexofenadine are available for use?
A: Fexofenadine is available as tablets, oral suspension, and raw powder. Healthcare professionals may use these forms to formulate personalized treatments depending on patient needs and application requirements.Q: Where should Fexofenadine not be stored?
A: Avoid storing Fexofenadine in bathrooms or any humid environments, as moisture can compromise its quality and shelf life. Always keep it in dry, temperature-controlled areas.Q: What are the main benefits of using Fexofenadine for allergies?
A: Fexofenadine provides rapid and reliable relief from allergy symptoms like rashes, swelling, itching, and watery eyes without usually causing drowsiness, making it suitable for daytime use and helping patients maintain normal activities.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese