RO SUVASTATIN
Product Details:
- Storage Dry Place
- Solubility low solubility in water, with reported values around 0.33 to 0.427 mg/ml
- HS Code 29420090
- Taste Sweet
- Particle Size 100-300 m
- Shelf Life 3 Years
- Ph Level pH 5,pH 6 and 7
- Click to View more
RO SUVASTATIN Price And Quantity
- 17000.00 INR/Kilograms
- 1 Kilograms
RO SUVASTATIN Product Specifications
- 481.539 g/mol Grams (g)
- NA
- not more than 20 ppm" for heavy metals
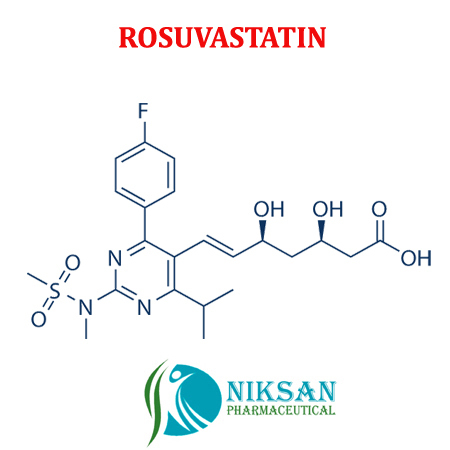
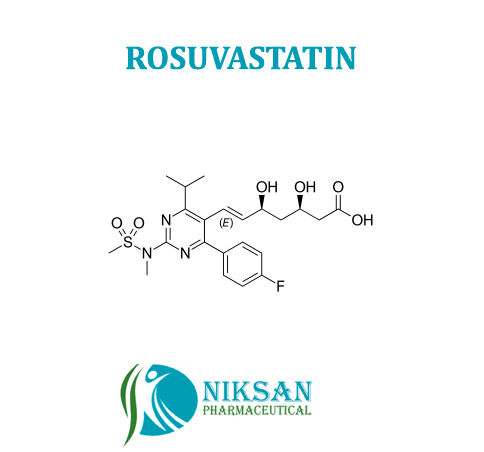
- ROSUVASTATIN
- C22H28FN3O6S
- 99 %
- 745.6C
- (E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate
- Powder
- C22H28FN3O6S
- No Smell
- 917-366-0
- Rosuvastatin belongs to a group of drugs "statins which is used in diet of bad cholesterol and fats. It alsoincreases good cholesterol in the blood.
- 147098-20-2
- 156-160C
- Pharmaceutical Intermediates
- 3 Years
- White to Off-White
- 100-300 m
- pH 5,pH 6 and 7
- Other
- low solubility in water, with reported values around 0.33 to 0.427 mg/ml
- Dry Place
- Sweet
- 29420090
RO SUVASTATIN Trade Information
- Mundra and Mumbai
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical is among worlds top most manufacturers,supplier, exporter and trader of the Rosuvastatinfinished formulations moreover Rosuvastatin API. Niksan Pharmaceutical is thehuge manufacturer, exporter and supplier of Rosuvastatin API and formulationsituated in Ankleshwar, Gujarat, India.
Niksan Pharmaceutical provides API and finished formulation of Rosuvastatin in many Indian states likeHimachal Pradesh, Andhra Pradesh, Tamil Nadu, Telangana, Kerala, Karnataka,Gujarat, Jammu & Kashmir, Chandigarh, Punjab, Uttarakhand, Chhattisgarh,Haryana, Maharashtra, Odisha, Uttar Pradesh, Delhi, Rajasthan, Madhya Pradesh,West Bengal, Bihar, Assam and many other Indian states.
Niksan Pharmaceutical is also huge exporter of the API andfinished formulations of Rosuvastatinin many Countries like Canada, Philippines, Trinidad & Tobago, Hungary,Nepal Australia, Lebanon, Sri Lanka, Finland, Pakistan, Puerto Rico,Bangladesh, United States, United Arab Emirates, Vietnam, Iraq, South Korea, Nigeria, Jordan, Switzerland,Slovenia, Hong Kong, Malaysia, Saudi Arabia, United Kingdom, Israel, Sweden,Germany, Greece, Egypt, Czechia, Thailand, Indonesia, South Africa, Denmark,Romania and many other countries.
Rosuvastatin belongs to a group of drugs "statins which is used in dietof bad cholesterol and fats. It alsoincreases good cholesterol in the blood.
SYNONYMS: Rosuvastatin,Rosuvastatina
IUPAC NAME: (3R, 5S, 6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid
CAS NO: 287714-41-4
FORMULA: C22H28FN3O6S
MOLECULAR MASS: 481.53 g/mol
STORAGE OF Rosuvastatin: Store incool and dry place. Keep medicine away from the reach of children and pets. Donot store this medicine in bathroom or any humidly place.
APPLICATIONS OF Rosuvastatin:Rosuvastatinis used in diet of bad cholesterol and fats. It is also increase goodcholesterol in the blood.
HOW TO USE: Take one tablet in one day. You can take Rosuvastatinevery day at any time.
Take this medication with or without food.
HOW RosuvastatinWORKS: Rosuvastatin reducing theamount of cholesterol which made by liver. Which can helps you in good diet.
CONTRAINDICATIONS:If you are a patient of liver problem or liverfailure kindly avoid this medication. If you have a chronic kidney disease or metabolic syndrome x do not take this medication. Kindlyavoid Rosuvastatin if you have recent operation and major traumatic injury.
PHARMACOKINETICS OF Rosuvastatin:Approximately 20% Rosuvastatin absorbed after oral administration. Half life ofRosuvastatin is approximately 19 hours. Rosuvastatin is not widely metabolized. Maximum time of Peak plasmaconcentration of Rosuvastatin is 3 to 5 hours. Approximately 78% Rosuvastatin isexcreted via bile and 28% excreted by renal excretion.
SIDE EFFECTS OF Rosuvastatin: Many people use this drug but very few side effects areseen in the patient. In the rare case Rosuvastatin cause worsen diabetes. Thisdrug may rarely cause muscle problem like muscle pain and weakness.
PRECAUTION: During the pregnancy do not use Rosuvastatin it may harm and unborn baby. Consultwith your doctor before the breast-feeding. Donot take alcohol and do not driving during the dose. Do not take thismedication if you have any liver problem.
CDSCO APPROVAL: Rosuvastatin Calcium eq. to Rosuvastatin20mg/20mg + Fenofibrate BP 67.5mg/145mg Tablets are approved by CDSCO in Indiain 24.08.2011
RosuvastatinCalcium Tablet (Additional Indication) is approved by CDSCO in India in 24.05.2010
RosuvastatinCalcium IP eq to Rosuvastatin 5mg + Ezetimibe 10mg. (Additional Strength) isapproved by CDSCO in India in 05.12.2011
Rosuvastatin5/10/20mg + Fenofibrate 67/145/160mg is approved by CDSCO in India in 23.08.2010
RosuvastatinCalcium tablets 5/10/20/40mg (Addl. Indication) is approved by CDSCO in Indiain 08.11.2008
RosuvastatinCalcium IP 5mg/10mg + Fenofibrate BP 160mg/160mg tablet is approved by CDSCO inIndia in 29.12.2010
Rosuvastatincalcium tablet is approved by CDSCO in India in 2003
Aspirin(EC Tablets) 75mg/75mg/150mg + Rosuvastatin (Granules) 5mg/10mg/10mg Capsules is approvedby CDSCO in India in 30.03.2011
RosuvastatinCalcium Tablets 5/10/20mg (Additional Indication) is approvedby CDSCO in India in 05.04.2011
FORMULATIONS AVAILABLE IN MARKET:
Rosuvastatin10 MG tablets
Rosuvastatin20 MG tablets
Rosuvastatin40 MG tablets
Rosuvastatin5 MG tablets
Rosuvastatin30 MG tablets
Rosuvastatin15 MG tablets
Rosuvastatin10 MG+Aspirin 75 MG tablets
Rosuvastatin5 MG+Fenofibrate 67 MG tablets
Rosuvastatin10 MG+Fenofibrate 67 MG tablets
Clopidogrel75 MG +Rosuvastatin 10 MG tablets
Fenofibrate67 MG+Rosuvastatin 20 MG tablets
Clopidogrel75 MG+Rosuvastatin 20 MG tablets
Aspirin 75MG+Rosuvastatin 20 MG tablets
Clopidogrel75 MG+Rosuvastatin 5 MG tablets
Rosuvastatin10 MG+Ezetimibe 10 MG tablets
Note: Product protected by valid patents are not offered for sale incountries where such patents are still valid and its liability is at BuyersRisk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese