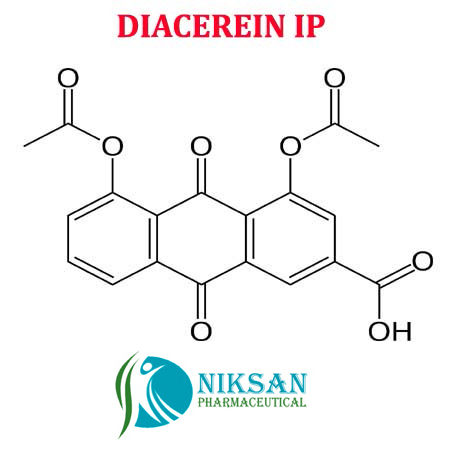
DIACEREIN
Product Details:
- Shelf Life 3 Years
- Molecular Formula C19H12O8
- Loss on Drying 0.5%
- Ph Level pH of 7
- Boiling point 631.5 C
- EINECS No 237-310-2
- HS Code 29420090
- Click to View more
DIACEREIN Price And Quantity
- 10 Kilograms
DIACEREIN Product Specifications
- 368.29 g/mol Grams (g)
- Medicine Grade
- Other
- Other
- Powder
- NA
- DIACEREIN
- Room Temperature
- 270 to 510 nm
- 217C to 245C
- poorly soluble in water, with a solubility of approximately 0.010 mg/mL
- 13739-02-1
- White to Off-White Solid
- Diacerein is one type of anthraquinone class of medicine. Diacerein normally used in joint pain related treatment. Diacerein used in conditions like gout, osteoarthritis, joint pain, and also used in the treatment of swollen joints, bones and stops the degradation of muscles.
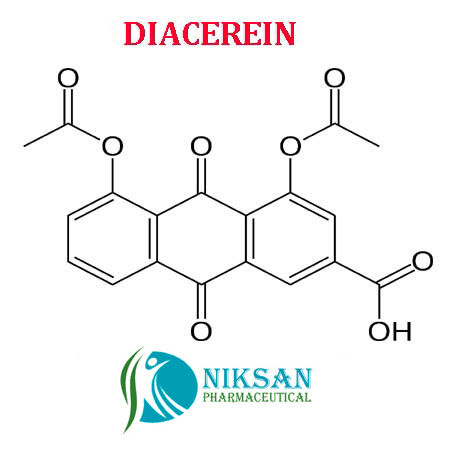
- C19H12O8
- 29420090
- pH of 7
- 631.5 C
- 237-310-2
- 0.5%
- Other
- 3 Years
- C19H12O8
- 99 %
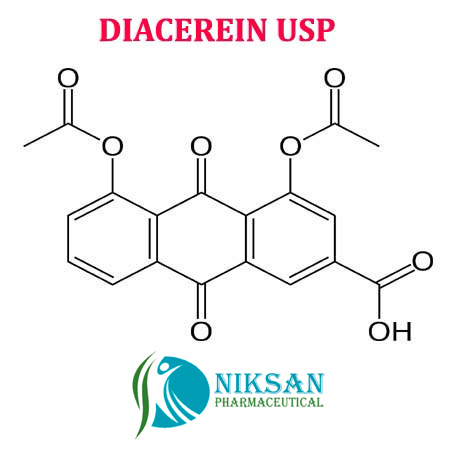
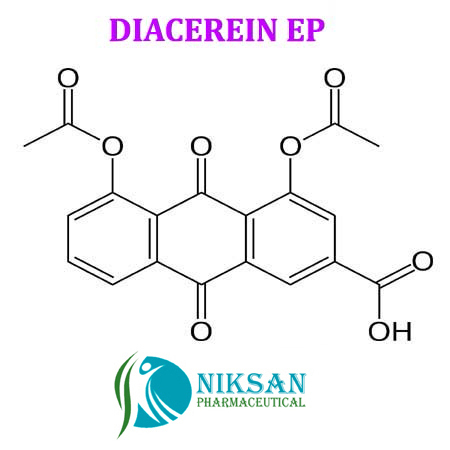
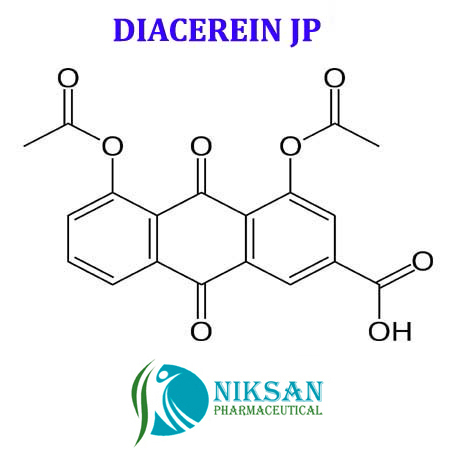
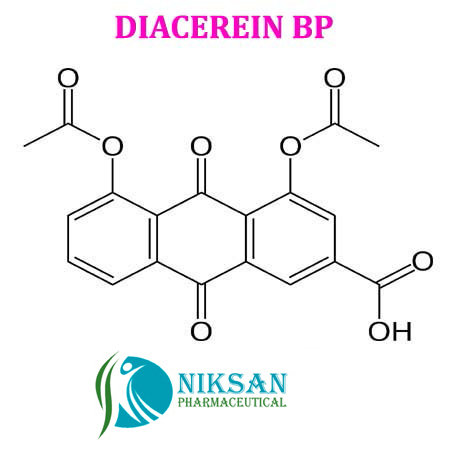
- 4,5-Bis(acetyloxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic acid
DIACEREIN Trade Information
- SAHAR AIR CARGO
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Chandigarh, Himachal Pradesh, Tripura, Manipur, Meghalaya, Uttarakhand, Daman and Diu, Dadra and Nagar Haveli, Lakshadweep, South India, East India, West India, Andaman and Nicobar Islands, Assam, Arunachal Pradesh, Bihar, Chhattisgarh, Delhi, Gujarat, Goa, Haryana, Jammu and Kashmir, Jharkhand, Karnataka, Madhya Pradesh, Maharashtra, Mizoram, Nagaland, Odisha, Punjab, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, West Bengal, Uttar Pradesh, North India, Telangana, Andhra Pradesh, Kerala, Central India, All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan pharmaceuticalis also large exporter of the API and finished pharmaceutical products ofDiacereinin many countries for years. The countries where we exporting are Myanmar (Burma), Nepal, Vietnam, Pakistan, India, Bangladesh, Egypt, Kuwait, Greece, Israel ,Malaysia, Thailand, Indonesia, Austria, Australia, Brazil, Germany, United Kingdom, United States, Japan and many more countries.
Diacereinis one type of anthraquinonoid class of medicine. Diacerein normally used in joint pain related treatment. Diacereinu sed in conditions like gout, osteoarthritis ,joint pain, and also used in the treatment of swollen joints, bones and stops the degradation of muscles.
SYNONYMS:Diacerein,
IUPACNAME:4, 5-bis (acetyloxy)-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid
CASNO: 13739-02-1
FORMULA:C19H12O8
368.29gram/mol
STORAGECONDITIONS:Storein cool and dry place. Place medicine away from direct heat and sun light. Do not put medicine in humid place. Keep away from children and pets.
HOWTO USE DIACEREIN:Take medication orally with or without food. If you have any confusion, kindly contact your doctor. Take medication regularly for the more benefits.
HOWDIACEREINWORKS:Diacerein reduces the joint pain byin hibiting the interleukin-1 beta-receptor. Diacerein also decreases the inflammation and also corrects the altered osteoblast activity.
PHARMACOKINETICS:Only25% of Diacerein absorbed after the oral administration. The bio avaibility of Diacerein is between 50-60%. The biological half-life Diacerein is 4-10 hrs. Almost 53% of Diacerein is excreted by faces and 37% of Diacerein eliminated by the urination.
SIDEEFFECTSOF DIACEREIN: The most common side effects of Diacerein are GI problems. Nausea, vomiting and discomfort of stomach also seenin some patients. The other side effects like soft stool and loose motion alsoseen in the patients. The other side effects like ab normality of liver and kidney functions are the major side effects of Diacerein.
PRECAUTIONS:Tell your doctor if you have allergic reaction to the Diacerein. If you have any disease like heart disease, kidney problem, liver problem, GI problem tell your doctor before writing prescription. The capsules should be swallowed whole without opening or breaking. If you are pregnant kingly avoid taking the medication.
CDSCOAPPROVAL:Celecoxib 100/200mg +Diacerein50/50mg approved by CDSCO in Indian in 19.07.2010,
Diacerein Modified Release100mg Capsule approved by CDSCOin Indian in 20.11.2009.
FORMULATIONSAVAILABLE IN MARKET:
Diacerein 50mg capsules
Diacerein 50mg + Curcumin 50mg capsules
Diacerein 50mg + Celecoxib 100 tablets
Diacerein 50mg + Celecoxib 200 tablets
Diacerein 50mg + Aceclofenac 100mg tablets
Note:Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
How Diacerein Supports Joint Health
Diacerein is classified within the anthraquinone group, known for its effectiveness in treating joint pain related conditions such as gout and osteoarthritis. By slowing the degradation of cartilage and muscle tissue in the joints, Diacerein provides long-term relief from pain and swelling. This makes it an excellent choice for individuals seeking pharmaceutical assistance for sustained joint mobility.
Safe Usage and Dosage Recommendations
Diacerein is formulated for oral intake in either tablet or capsule form. The dosage should adhere to your doctors instructions, whether with or without food. For any uncertainties about timing or dosage adjustments, consulting a healthcare professional is advised. Following the correct routine can maximize therapeutic benefits and minimize potential risks.
Optimal Storage Ensures Potency
To ensure Diacerein maintains its purity and effectiveness, store the powder in a tightly sealed container at room temperature. Choose a cool, dry location, protected from direct sunlight and heat sources. Such storage practices prevent chemical degradation and help you benefit from the full product shelf life of up to three years.
FAQs of DIACEREIN:
Q: How should Diacerein be stored to preserve its quality?
A: Store Diacerein in a well-closed container at room temperature, in a cool and dry place. Keep it out of direct sunlight and away from heat sources to maintain its stability and effectiveness.Q: What is the recommended method for taking Diacerein?
A: Diacerein is typically administered orally in the form of tablets or capsules. It can be taken with or without food. Always follow your doctors instructions regarding dosage and administration.Q: When is Diacerein commonly recommended for use?
A: Doctors prescribe Diacerein primarily for joint pain, osteoarthritis, gout, and conditions that involve swollen joints or the prevention of muscular degradation in the bones.Q: Where is Diacerein manufactured and who supplies it?
A: Niksan Pharmaceutical, based in India, manufactures, supplies, exports, and distributes Diacerein, ensuring high standards with GMP certification and a ready Drug Master File (DMF).Q: What is the process for converting Diacerein powder into medicine?
A: The raw Diacerein powder is processed in pharmaceutical facilities to produce standardized tablets and capsules, maintaining strict quality control to ensure safety and efficacy for end-users.Q: How does Diacerein benefit individuals with joint conditions?
A: Diacerein inhibits the breakdown of cartilage, reducing inflammation and swelling in joints. Regular use can improve mobility and delay further joint and muscle degradation, especially in osteoarthritis and gout.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese