DR OTAVERINE
Product Details:
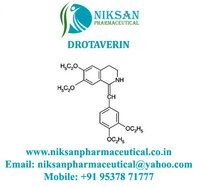
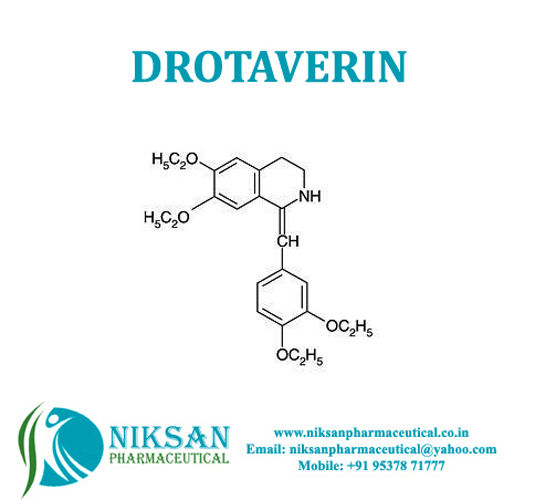
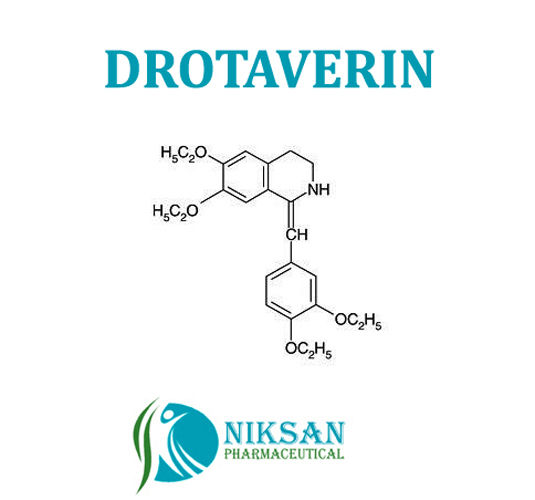
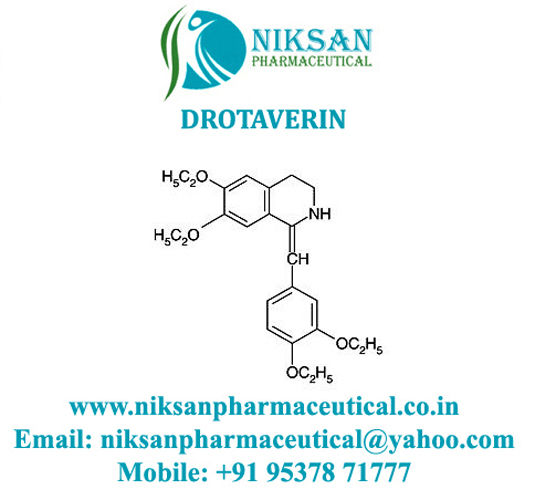
- Structural Formula C24H31NO4
- Heavy Metal (%) NA
- HS Code 29420090
- Taste Other
- Particle Size 5 8m
- EINECS No CAS 985-12-6
- Smell Other
- Click to View more
DR OTAVERINE Price And Quantity
- 5 Kilograms
- 3500.00 INR/Kilograms
DR OTAVERINE Product Specifications
- Medicine Grade
- 5 8m
- Pale Yellow to Yellow Solid
- 197 C
- CAS 985-12-6
- Other
- Other
- Room Temperature
- not more than 0.5%
- Other
- Drotaverine is one type of anti-spasmodic drug.Drotaverine is the posphodiesterase 4 inhibitor. Drotaverine used to enhance the cervical dilation during the birth process.Drotaverine also treat the spasm and twitches the smooth muscle of the stomach.
- C24H31NO4
- 586.1C
- 99 %
- DROTAVERINE
- Other
- sparingly soluble in water
- 3 Years
- DROTAVERINE
- C24H31NO4
- 29420090
- NA
- 397.50 g/mol
- between 3.5 and 5.5
- 14009-24-6
DR OTAVERINE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO GMP,GMP,GLP,ISO
Product Description
is words top leading manufacturer, exporter, trader and supplier of DrotaverineAPI as well as finished pharmaceutical products of Drotaverineamong the pharmaceutical companies. Our product Drotaverineis widely used and appreciated by our group companies and also ourcustomers and users all around the nations. We offer the Drotaverinein very affordable price.
Niksan Pharmaceutical provides API and finishedformulations of Drotaverine in allover Indian states Like Kerala, Gujarat, Haryana, Rajasthan, Madhya Pradesh,Uttar Pradesh, Rajasthan, Karnataka, Meghalaya, Tamilnadu, Goa, Sikkim, Assam,Punjab, Delhi, Bihar, Jammu Kashmir Etc.
Niksan Pharmaceutical is also large exporter ofthe API and finished pharmaceutical products of Drotaverine in many countries for years. The countries where weexporting are Myanmar (Burma), Nepal, Afghanistan, Uganda, Ghana, Yemen,Pakistan, Syria, Lithuania, Vietnam, Iraq, Nigeria, Thailand, Malaysia,Hungary, Romania, Egypt, Bolivia, Ukraine, Poland, United Kingdom, MexicoRussia, Germany, Canada, Turkey, United States, Spain, France and many morecountries.
Drotaverine is one type ofanti-spasmodic drug. Drotaverine is the posphodiesterase 4 inhibitor.
Drotaverine used to enhance thecervical dilation during the birth process. Drotaverine also treat the spasmand twitches the smooth muscle of the stomach.
SYNONYMS OF DROTAVERINE: Drotaverin, Drotaverina, Drotaverine,Drotaverinum.
IUPAC NAME: (1Z)-1-[(3, 4-diethoxyphenyl) methylidene]-6,7-diethoxy-1, 2, 3, 4-tetrahydroisoquinoline.
CAS NO:14009-24-6
FORMULA:C24H31NO4
MOLECULAR MASS: 397.50 g/mol
STORAGE CONDITIONS: Store in room temperature in dry place. Donot put it in bathroom or fridge. Keep away from direct light and heat. Keepaway from children and pet.
HOW TO USE: Take the medicine orally with or withoutfood. If you have any confusion regarding to the medication ask your doctor. Takemedication regularly to achieve maximum benefits from it.
HOW DROTAVERINEWORKS: Drotaverine is anti-spasmodic drug which inhibits the phosphodiesterase4 enzyme and by this it cause vasodilation of the cervical muscle whiles thebirth process. It is also used in the treatment of headache and syndrome.
PHARMACOKINETICS: Drotaverine inhibits the phosphodiesterase4 enzyme and increase the cAMP concentration. The bioavaibility of Drotaverineis variable. About 80-95% the Drotaverine drug binds with the plasma protein. Thehalf-life of Drotaverine is between 7-12hrs. The elimination of the Drotaverineis done mainly by the bile.
SIDE EFFECTS: The side effects ofDrotaverine are nausea, vomiting, swelling of face, lips, tongue. Drotaverinealso cause dry mouth, change in pulse rate, Dizziness, headache.
PRECAUTIONS: Tell your doctor if youhave allergic reaction from the Drotaverine. If you have past disease likeliver disease, kidney problem or heart related problem kindly consult and tellyour doctor. Do not take alcohol or marijuana while taking the medication. Donot overdose the medication it can cause many side effects.
CDSCO APPROVAL: Drotaverine + Aceclofenac (80mg + 100mg)Tablets approved by CDSCO in Indian in 15.09.2008.
FORMULATIONSAVAILABLE IN MARKET:
Drotaverine 40mg tablets
Drotaverine 80mg tablets
Drotaverine 40mg/2ml oral solution
Drotaverine 20mg/5ml oral solution
Drotaverine 10mg/5ml oral solution
Drotaverine 80mg + Diclofenac 50mg tablets
Drotaverine 40mg + Nimesulide 100mg tablets
Drotaverine 80mg + Aceclofenac100mg tablets
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese