ACITIVE PHARMACEUTICAL INGREDIENTS CILNIDIPINE
Product Details:
- Molecular Formula C27H28N2O7
- EINECS No 132203-70-4
- Ph Level 7
- Melting Point 110
- Boiling point 652.6
- HS Code 29420090
- Heavy Metal (%) 10 PPM
- Click to View more
ACITIVE PHARMACEUTICAL INGREDIENTS CILNIDIPINE Price And Quantity
- 6000.0 INR/Kilograms
- 5 Kilograms
ACITIVE PHARMACEUTICAL INGREDIENTS CILNIDIPINE Product Specifications
- Room Temperature
- 0.5
- C27H28N2O7
- 5 Years
- SOLUBLE IN PHARMACEUTICAL SOLVENT
- Woody
- 132203-70-4
- 99.8 %
- CILNIDIPINE
- 652.6
- 110
- 7
- 29420090
- CILNIDIPINE
- C27H28N2O7
- 132203-70-4
- Odorless
- YELLOW POWDER
- Cilnidipine comes under the calcium channel blocker class of medicine. Most of it is used as anti-hypertensive agent. Cilnidipine have compatibility with other API like Telmisartan, Olmesartan Medoxomil, Ramipril and Metoprolol Succinate. Cilnidipine can act on the N-type calcium channel in addition to acting on the L-type calcium channel.Cilnidipine dilates both arterioles and venules, reducing the pressure in the capillary bed.
- Cardiovascular Agents
- 492.52 Grams (g)
- Powder
- 10 PPM
- 20 MICRON
- Medicine Grade
ACITIVE PHARMACEUTICAL INGREDIENTS CILNIDIPINE Trade Information
- INDIA
- Cheque, Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Within a certain price range free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Middle East, Africa, Asia
- Punjab, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal, Jharkhand, Odisha, All India, South India, Central India, West India, North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Chhattisgarh, Haryana, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura
- FDCA, GMP, GLP AND ISO
Product Description
NIKSAN PHARMACEUTICAL providesCilnidipineto all states of India like Punjab, Rajasthan, Andhra Pradesh, Haryana, Telangana, Bihar, Karnataka, Delhi, Tamil Nadu, Odisha, Maharashtra, West Bengal, Uttar Pradesh, Gujarat, and Madhya Pradesh etc. We also give gift samples to the companies for R&D purpose.Cilnidipinecomes under the calcium channel blocker class of medicine. Most of it is used a santi-hypertensive agent.
Cilnidipine have compatibility with other API like Telmisartan, Olmesartan Medoxomil, Ramipril and Metoprolol Succinate. Cilnidipine can act on theN-type calcium channel in addition to acting on theL-type calcium channel. Cilnidipinedilates both arterioles and venues, reducing the pressure in the capillary bed.
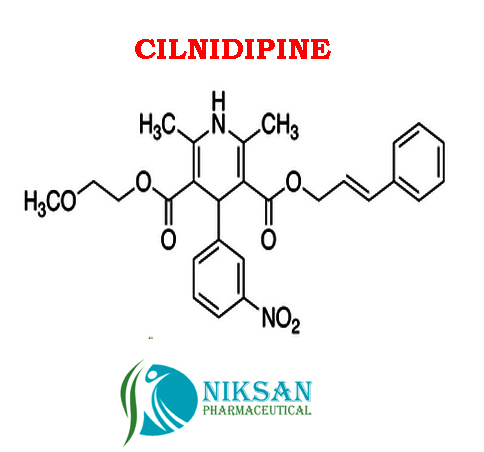
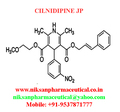
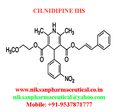
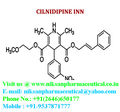
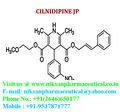
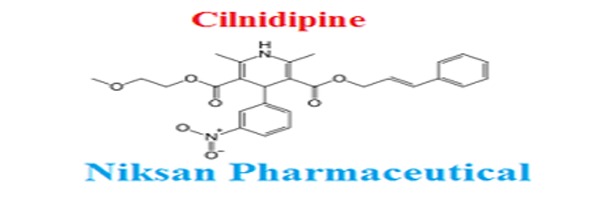
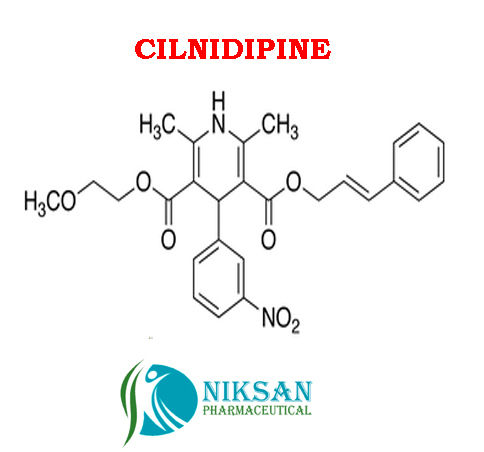
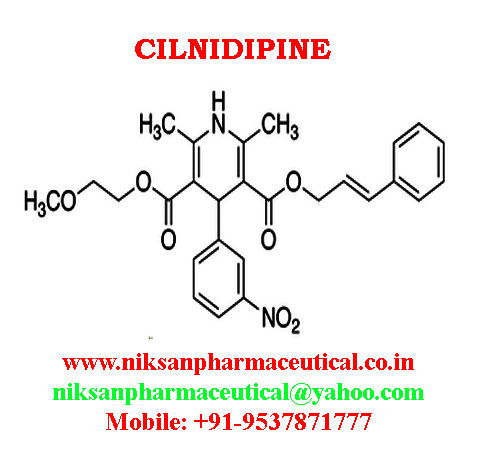
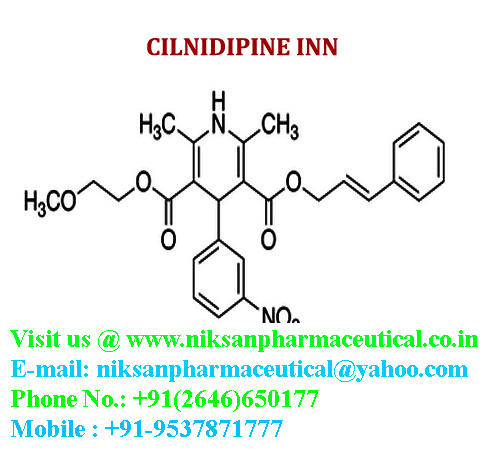
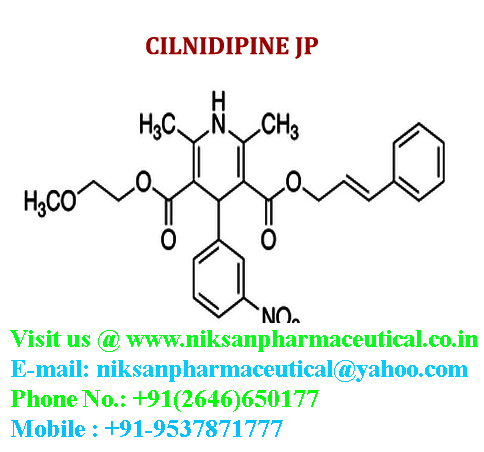
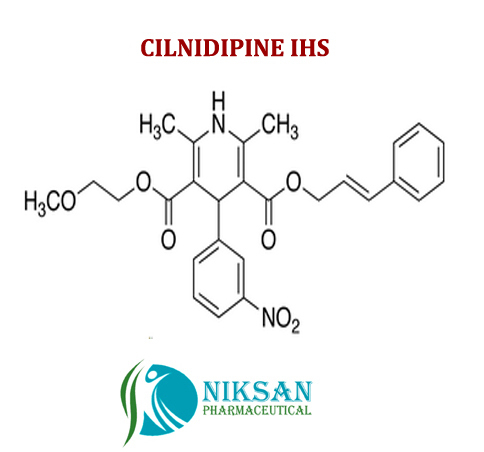
IUPAC NAME:3-(2-methoxyethyl)5-(2E)-3-phenylprop-2-en-1-yl2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
CASNO:132203-70-4
FORMULA:C27H28N2O7
MOLECULAR MASS:492.52 g/mol
STORAGE:Store the medicine in cool and dry place. Store it away from the direct sun light. Do not change packet of the Cilnidipine tablet. Keep away from reach of children and pets.
APPLICATION:Cilnidipine is primarily used for the treatment of high blood pressure also referred to as hyper tension. The Cilnidipine have the calcium channel blocker activity it is also used in the treatment of headache, vision related problems, irregular heartbeat, chest pain, and fatigue.
HOW TO USE:Simply take the medicine as per your doctor says. The tablet should be taken orally with water. The tablet must be swallowed whole without breaking or chewing.
HOW CILNIDIPINE WORKS:Cilnidipine blocks the calcium channels in blood vessels. By this the blood supplies towards the heart increase and by that the hyper tension decreases.
CONTRAINDICATIONS:Do not take Cilnidipine medicine in pregnancy and lactation period. Do not give medicine to the patient under 18 years. If you have any confusion related to the medicine consult to your doctor.
PHARMACOKINETICS: Cilnidipine is one type of lipophilic drug. So mainly itis metabolites in the liver or kidneys. Cilnidipine is rapidly absorbed in the blood plasma in only 2hrs . Cilnidipine normally excreted by urination.
SIDE EFFECTS:There are some common side effects of the Cilnidipine are Dizziness, hypotension, swelling of (face, eyes, tongue, and feet), fast heartbeats, muscle pain, stomach pain, skin rash.
PRECAUTIONS:Dont take medicine if you are allergic to Cilnidipine. If you have any heart or liver problem kindly find some alternative of Cilnidipine. The effect of Cilnidipine is not known yet so do not take it if you are breast feeding the baby. Do not share the medicine with other people.
CDSCO APPROVAL: Cilnidipine tablet 5mg approved by CDSCO in India in21.06.2007.
Cilnidipine tablet 10 mg is approved by CDSCO in 21.06.2007.
FORMULATIONSIN MARKET:
Cilnidipine 5 mg Tablets
Cilnidipine 10 mg Tablets
Cilnidipine 20 mg Tablets
Cilnidipine 5 mg +Telmisartan 20 mg Tablets
Cilnidipine10 mg +Telmisartan 40 mg Tablets
Cilnidipine 10 mg +Metoprolol Succinate 25mg Tablets
Cilnidipine 10 mg +Metoprolol Succinate 50mg Tablets
Cilnidipine 10 mg +Olmesartan Medoxomil 20mg Tablets
Cilnidipine 10 mg +Olmesartan Medoxomil 40mg Tablets
Cilnidipine 10 mg +Ramipril IP 10 mg Capsules
Cilnidipine 10 mg +Ramipril IP 2.5 mgCapsules
Cilnidipine 5 mg +Hydrochlorothiazide 12.5mg + Telmisartan 40 mg capsules
Note:Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese