SILODOSIN INTERMEDIATE
Product Details:
- Storage 28C, protect from light and moisture
- Solubility Stable under recommended storage conditions
- Molecular Formula C11H11F3N2O
- Melting Point 95105 C
- Chemical Name (R)-5-(Aminomethyl)-2-(2,2,2-trifluoroethoxy)-1H-indole
- CAS No 160970-57-0
- Appearance White to off-white crystalline powder
- Click to View more
SILODOSIN INTERMEDIATE Price And Quantity
- 6000.00 INR/Kilograms
- 10 Kilograms
SILODOSIN INTERMEDIATE Product Specifications
- White to off-white crystalline powder
- 95105 C
- Powder
- (R)-5-(Aminomethyl)-2-(2,2,2-trifluoroethoxy)-1H-indole
- Stable under recommended storage conditions
- 28C, protect from light and moisture
- C11H11F3N2O
- 160970-57-0
SILODOSIN INTERMEDIATE Trade Information
- ANY INDIAN AIR PORT
- 2000 Kilograms Per Month
- 10 Days
- Yes
- Free samples are available
- HDPE DRUM WITH 2 INNER LDPE LINNER
- Dadra and Nagar Haveli, Chandigarh, Himachal Pradesh, Andaman and Nicobar Islands, Uttarakhand, Daman and Diu, Lakshadweep, South India, East India, West India, Assam, Arunachal Pradesh, Bihar, Delhi, Gujarat, Goa, Jammu and Kashmir, Jharkhand, Karnataka, Madhya Pradesh, Maharashtra, Mizoram, Meghalaya, Manipur, Punjab, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, West Bengal, Nagaland, Haryana, Uttar Pradesh, North India, Andhra Pradesh, Kerala, Central India, Odisha, Chhattisgarh, All India
- FDA, GMP, GLP, ISO
Product Description
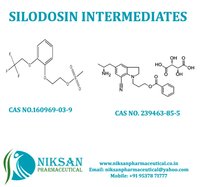
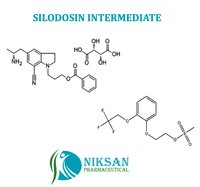
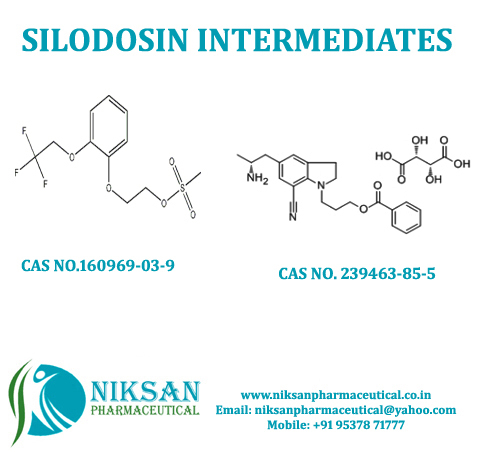
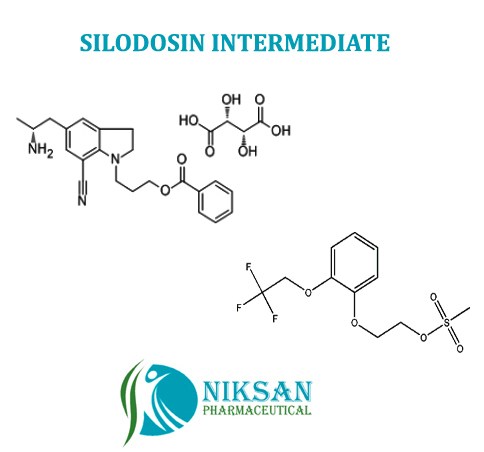
Niksan Pharmaceutical manufacturer, exporterand supplier of a wide range of 2-[2-(2,2,2-Trifluoroethoxy) phenoxy] ethyl methanesulfonate and5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile(2R,3R)-2,3-dihydroxybutanedioate.Itsmainly use in manufacturing of SILODOSIN. Niksan Pharmaceutical is having MP,GLP manufacturing facility in heart of pharmaceutical in Ankleshwar, Gujarat,India
Chemical Name: 2-[2-(2,2,2-Trifluoroethoxy)phenyl]ethyl methane sulfonate
CAS No: 160969-03-9
Molecular Formula: C11H13F3O5S
Molecular Weight: 314.28
Chemical Name: 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile(2R,3R)-2,3-dihydroxybutanedioate
CAS No: 239463-85-5
Molecular Formula: C26H31N3O8
Molecular Weight: 513.53
Application: Used in SILODOSIN
Superior Quality Silodosin Intermediate
Our Silodosin intermediate is produced using advanced pharmaceutical processes, ensuring high purity and stability. With strict adherence to GMP and GLP standards, we deliver consistent results batch after batch. Niksan Pharmaceutical takes pride in supplying intermediates that meet international regulatory requirements and support reliable production of Silodosin.
Versatile Pharmaceutical Applications
This intermediate is suitable for various pharmaceutical formulations, including tablets, capsules, injections, ointments, and syrups. Its universal solubility and stable properties make it a preferred choice for manufacturers seeking efficiency in production and flexibility across diverse drug dosage forms.
Strict Storage and Handling Guidelines
The Silodosin intermediate should be stored in a well-closed container at temperatures between 28C, protected from light and moisture. Adhering to these guidelines ensures optimal chemical stability and protects the integrity of the raw material during handling, storage, and transportation.
FAQs of SILODOSIN INTERMEDIATE:
Q: How is the SILODOSIN INTERMEDIATE used in pharmaceutical manufacturing?
A: SILODOSIN INTERMEDIATE serves primarily as a crucial raw material in the synthesis of Silodosin, which is then processed into final pharmaceutical dosage forms such as tablets, capsules, injections, ointments, and syrups.Q: What dosage forms can be prepared using SILODOSIN INTERMEDIATE?
A: The intermediate is suitable for preparing various pharmaceutical forms, including tablets, capsules, injections, ointments, and syrups, providing flexibility to manufacturers in their production processes.Q: When should the SILODOSIN INTERMEDIATE be stored and handled with extra care?
A: Always store and handle the intermediate in a well-closed container, maintained at 28C and protected from light and moisture, to preserve its stability and prevent any degradation.Q: Where is the SILODOSIN INTERMEDIATE manufactured and supplied from?
A: It is manufactured and supplied by Niksan Pharmaceutical from their state-of-the-art facility located in Ankleshwar, Gujarat, India, following strict GMP and GLP standards.Q: What is the process of ordering this intermediate in quantities like 100 units?
A: To order, contact Niksan Pharmaceutical directly through their distributor, exporter, importer, manufacturer, supplier, or trader channels. Custom quantities and dosage strengths are available as per client requirements.Q: How does the SILODOSIN INTERMEDIATE benefit pharmaceutical manufacturers?
A: By providing a stable, high-purity intermediate that complies with international quality standards, manufacturers can rely on efficient synthesis of Silodosin, ensuring consistent and effective end products.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Pharma Intermediates' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese











![2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate](https://cpimg.tistatic.com/05818657/b/4/2-2-2-2-2-Trifluoroethoxy-phenoxy-ethyl-methanesulfonate.jpg?tr=w300)



