DAPAGLIFLOZIN
Product Details:
- Particle Size 100.13 7.2 to 399.08 2.4 nm
- Ph Level 1.2 to 6.8
- Shelf Life 3 Years
- Melting Point 65-70C
- Heavy Metal (%) NA
- EINECS No not available
- Solubility practically insoluble in water, slightly soluble in ethanol, and practically insoluble in hexane
- Click to View more
DAPAGLIFLOZIN Price And Quantity
- 1 Kilograms
DAPAGLIFLOZIN Product Specifications
- not available
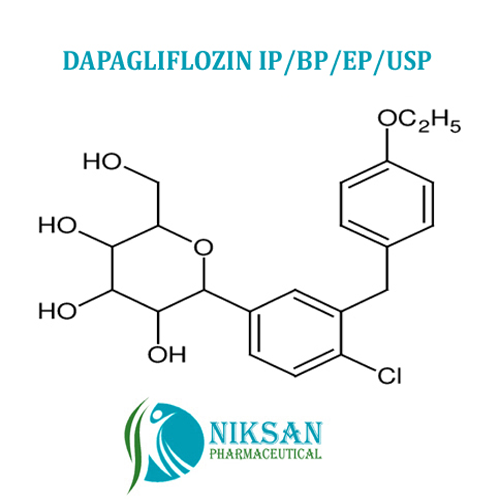
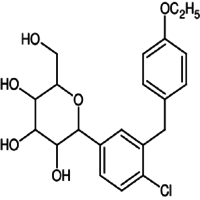
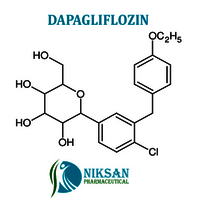
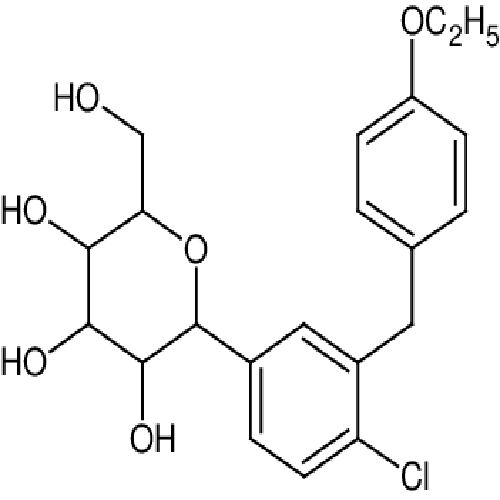
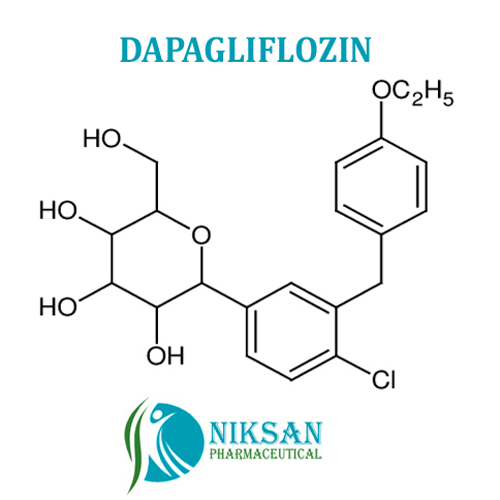
- (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
- Powder
- practically insoluble in water, slightly soluble in ethanol, and practically insoluble in hexane
- not more than 1.0% w/w
- Other
- 100.13 7.2 to 399.08 2.4 nm
- Sweet
- 1.2 to 6.8
- Room Temperature
- 609 C
- Medicine Grade
- 29329900
- 98%
- Pharmaceutical Intermediates
- white to off-white
- DAPAGLIFLOZIN
- 461432-26-8
- 408.873 g/mol Kilograms (kg)
- 3 Years
- C21H25ClO6
- 65-70C
- NA
- C21H25ClO6
DAPAGLIFLOZIN Trade Information
- Nhava-Sheva
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Days
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia, Australia, Central America, North America, South America, Western Europe, Middle East, Africa
- WHO GMP,GMP,GLP,ISO
Product Description
is one of the biggest manufacturer, supplier, exporter and trader ofthe DAPAGLIFLOZINfinished formulations moreover Dapagliflozin API. Niksan Pharmaceutical is the huge manufacturer, exporter andsupplier of Dapagliflozin API and formulation situated in Ankleshwar, Gujarat, India.
NiksanPharmaceutical is the suppliers, manufacturer, exporters and trader of
Dapagliflozin inthedomestic level as well as the international market.
Niksan is anIndian based pharmaceutical company so it also supplies and manufactures the Dapagliflozin to allIndian states like Gujarat, Maharashtra, Punjab, Delhi, Tamilnadu, Goa, UttarPradesh, Karnataka, Jammu & Kashmir, West Bengal, Assam, Rajasthan, Hyderabad,Karnataka, Kerala, Madhya Pradesh, Manipur, Meghalaya, Mizoram, Nagaland,Orissa, Punjab and Haryana.
NiksanPharmaceutical and Niksan group companies are exporters, suppliers andmanufacturers of the API and also Dapagliflozin formulations in many countries for manyyears. Niksan pharmaceutical currently exports Dapagliflozin API and Formulation to countries like NewZealand, Jordan, Belgium, United Kingdom, Australia, Italy, Ireland, Hong Kong,Morocco,, Portugal, France, South Africa, Philippines, Malaysia, Singapore,Israel, Greece, Saudi Arabia, Egypt, Taiwan, Netherlands, South Korea,Switzerland, Algeria, Pakistan, Canada, Vietnam, United States, Germany, Braziland many other countries.
DAPAGLIFLOZINis an oral antihyperglycemicagent that belongs to the class of SGLT2 inhibitors. It is used for thetreatment of type 2 diabetes mellitus, and also helps in reducing the risk ofhospitalization for heart failure in patients with or without diabetes.
SYNONYSMS:Forxiga, Farxiga
IUPAC NAME: (2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-(propyl)phenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
CAS NO:461432-26-8
FORMULA:C21H25ClO6
MOLECULAR MASS: 408.87 g/mol
STORAGE CONDITIONS:Store at room temperature, between 20C to25C (68F to 77F). Protect from moisture and light. Store in a tightly closedcontainer.
HOW TO USE:The recommendedstarting dose is 5 mg once daily, taken in the morning with or without food.The dose can be increased to 10 mg once daily based on individual tolerance andclinical response. Use only as prescribed by your physician.
HOW DAPAGLIFLOZINWORKS:Dapagliflozininhibits the sodium-glucose co-transporter 2 (SGLT2) in the proximal renaltubules, reducing the reabsorption of filtered glucose and increasing urinaryglucose excretion. This mechanism improves glycemic control and contributes toweight and blood pressure reduction.
PHARMACOKINETICSOF DAPAGLIFLOZIN:Dapagliflozin is rapidly absorbed after oral administration, with peakplasma concentrations occurring within 2 hours. It is extensivelyprotein-bound, metabolized primarily by UGT1A9 into inactive metabolites, andexcreted mostly via urine. The terminal half-life is about 12.9 hours.
SIDE EFFECTSOF DAPAGLIFLOZIN: The normal side effects caused by Dapagliflozin are Genital yeast infections, Urinary tractinfections, Increased urination, Thirst, Constipation, Hypoglycemia (especially when combined with insulin or sulfonylureas), Dehydration, Rare risk of diabetic ketoacidosis.
PRECAUTIONS: Avoid use in patientswith type 1 diabetes or diabetic ketoacidosis. Monitor renal functionperiodically. Caution should be taken in patients with low blood pressure,elderly patients, or those on diuretics. Use in pregnancy only if clearlyneeded.
CDSCOAPPROVAL:
Dapagliflozin Tablet 5mg/10mg 25.02.15
FORMULATIONSAVAILABLE IN MARKET:
DAPAGLIFLOZIN 5 MG Tablets
DAPAGLIFLOZIN 10 MG Tablets
DAPAGLIFLOZIN + METFORMIN Tablets
DAPAGLIFLOZIN + SAXAGLIPTIN Tablets
Note: Productprotected by valid patents are not offered for sale in countries where suchpatents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
High-Quality Medicine Grade Compound
Dapagliflozin is manufactured and supplied in medicine grade form, ensuring rigorous quality standards. With a purity of 98% and physical characteristics tailored for pharmaceutical synthesis, it is ideal for use in research and industrial medicine manufacturing. The structural formula and specified melting point support its stable application in varied processes.
Optimal Storage and Handling
Recommended storage for Dapagliflozin is at room temperature, which helps preserve its stability and extend its shelf life to up to three years. Its physical formpowderand specific particle size range make it straightforward to handle and incorporate into production systems. Strict loss on drying specifications guarantee consistent product quality.
Versatile Pharmaceutical Applications
As a pharmaceutical intermediate, Dapagliflozin is used in creating antidiabetic formulations. Its potent chemical profile and sweet taste support its acceptability and versatility in the medicine manufacturing landscape. Its appearance and solubility profiles also enable broad process compatibility, making it suitable for various formulation environments.
FAQs of DAPAGLIFLOZIN:
Q: How should Dapagliflozin be stored for optimal shelf life and quality?
A: It should be kept at room temperature in tightly sealed containers to maintain its physical and chemical properties, ensuring a shelf life of up to three years.Q: What are the primary uses of Dapagliflozin in the pharmaceutical industry?
A: Dapagliflozin serves as an intermediate compound for the synthesis of antidiabetic medications, supporting the development of drug formulations that help regulate blood sugar levels.Q: When is it appropriate to use Dapagliflozin in medicine manufacturing?
A: Dapagliflozin is incorporated during the production stages when antidiabetic agents are being synthesized, as its purity and stability are essential for medicine-grade applications.Q: Where is Dapagliflozin typically available for purchase or distribution?
A: It is predominantly distributed, exported, imported, and manufactured in India, commonly available through pharmaceutical suppliers and traders specializing in intermediates.Q: What is the process for handling Dapagliflozin in laboratory or industrial environments?
A: Given its powdered form and specified particle size, Dapagliflozin should be handled in controlled, dry environments using proper safety protocols to ensure product integrity and operator safety.Q: What are the benefits of Dapagliflozin for medicine manufacturers?
A: Manufacturers benefit from its high purity, stability, and consistent particle size, which facilitate reliable incorporation into pharmaceutical formulations and ensure effective drug synthesis.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Pharma Intermediates' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese