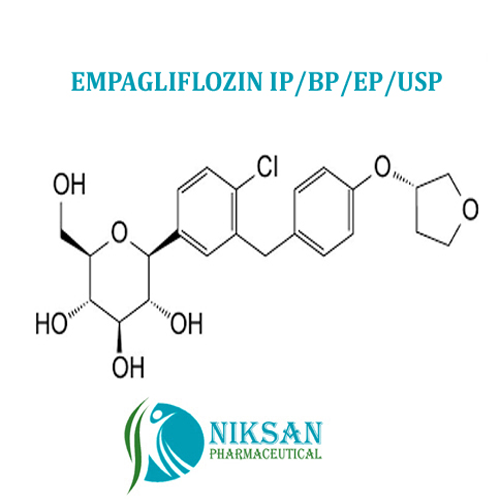
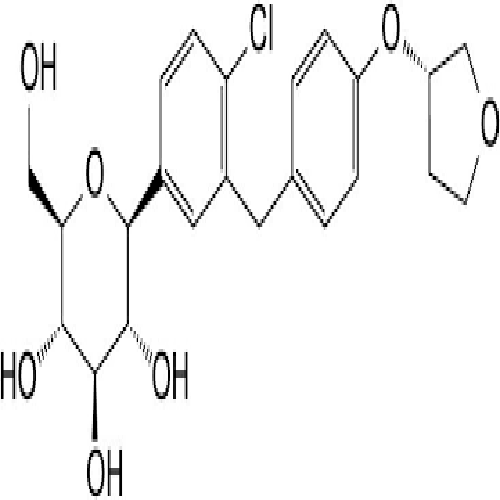
EMPAGLIFLOZIN
Product Details:
- EINECS No 620-176-8
- Boiling point 665C
- Molecular Formula C23H27ClO7
- Loss on Drying NA
- Ph Level pH 7.4
- Particle Size between 10 m and 100 m
- Shelf Life 3 Years
- Click to View more
EMPAGLIFLOZIN Price And Quantity
- 1 Kilograms
EMPAGLIFLOZIN Product Specifications
- C23H27ClO7
- 450.91 g/mol Grams (g)
- 864070-44-0
- Other
- white to yellowish
- Medicine Grade
- Powder
- C23H27ClO7
- 620-176-8
- 665C
- NA
- between 10 m and 100 m
- pH 7.4
- 3 Years
- 99.0%
- Other
- very slightly soluble in water, slightly soluble in acetonitrile and ethanol, sparingly soluble in methanol, and practically insoluble in toluene
- 30049099
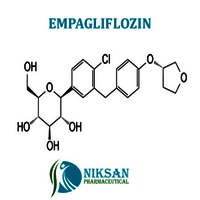
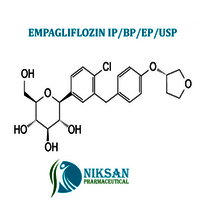
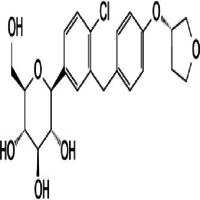
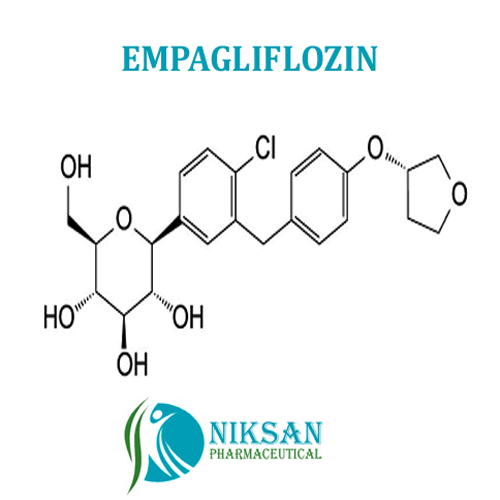
- (1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol
- Empagliflozin is used treat type 2 diabetes
- 150C to 155C
- less than 0.002%
- Pharmaceutical Intermediates
- Room Temperature
- EMPAGLIFLOZIN
EMPAGLIFLOZIN Trade Information
- Mumbai
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- WHO GMP,GMP,GLP,ISO
Product Description
is one of the biggest manufacturer, supplier, exporter and trader ofthe EMPAGLIFLOZIN finished formulationsmoreover EMPAGLIFLOZINAPI. Niksan Pharmaceutical is the huge manufacturer, exporter andsupplier of EMPAGLIFLOZINAPI and formulation situated in Ankleshwar, Gujarat, India.
NiksanPharmaceutical is the suppliers, manufacturer, exporters and trader of
EMPAGLIFLOZIN inthedomestic level as well as the international market.
Niksan is anIndian based pharmaceutical company so it also supplies and manufactures the EMPAGLIFLOZIN to allIndian states like Gujarat, Maharashtra, Punjab, Delhi, Tamilnadu, Goa, UttarPradesh, Karnataka, Jammu & Kashmir, West Bengal, Assam, Rajasthan, Hyderabad,Karnataka, Kerala, Madhya Pradesh, Manipur, Meghalaya, Mizoram, Nagaland,Orissa, Punjab and Haryana.
NiksanPharmaceutical and Niksan group companies are exporters, suppliers andmanufacturers of the API and also EMPAGLIFLOZIN formulations in many countries for manyyears. Niksan pharmaceutical currently exports EMPAGLIFLOZINAPI and Formulation to countries like New Zealand, Jordan,Belgium, United Kingdom, Australia, Italy, Ireland, Hong Kong, Morocco,,Portugal, France, South Africa, Philippines, Malaysia, Singapore, Israel,Greece, Saudi Arabia, Egypt, Taiwan, Netherlands, South Korea, Switzerland,Algeria, Pakistan, Canada, Vietnam, United States, Germany, Brazil and manyother countries.
EMPAGLIFLOZIN is an oralantihyperglycemic agent of the SGLT2 (sodium-glucose co-transporter 2)inhibitor class, used to improve glycemic control in adults with type 2diabetes mellitus. It also reduces the risk of cardiovascular death in patientswith type 2 diabetes and established cardiovascular disease.
SYNONYSMS:Jardiance
IUPAC NAME: (1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-D-glucitol
CAS NO:864070-44-0
FORMULA:C23H27ClO7
MOLECULAR MASS: 450.91 g/mol
STORAGE CONDITIONS:Store at 20C to 25C (68F to 77F).Protect from moisture and light. Store in a tightly closed container in a cool,dry place.
HOW TO USE:The usual startingdose is 10 mg orally once daily in the morning, with or without food. Based ontolerance and clinical response, the dose may be increased to 25 mg once daily.Always use as prescribed by the physician.
HOW EMPAGLIFLOZINWORKS:Empagliflozinworks by inhibiting SGLT2 in the proximal renal tubules. This reduces glucosereabsorption and increases urinary glucose excretion, thereby lowering bloodglucose levels. It also has beneficial effects on blood pressure and weight.
PHARMACOKINETICSOF EMPAGLIFLOZIN:Empagliflozin is rapidly absorbed after oral administration, with peakplasma concentrations occurring at 1.5 hours. It is 86% protein-bound,metabolized mainly by glucuronidation, and excreted via urine and feces.Terminal half-life is approximately 12.4 hours.
SIDE EFFECTSOF EMPAGLIFLOZIN:Urinarytract infections, Increased urination, Genital yeast infections, Dehydration, Hypotension, Increased cholesterol levels, Risk of ketoacidosis (rare), Acute kidney injury(rare)
PRECAUTIONS: Not for use inpatients with type 1 diabetes or diabetic ketoacidosis. Monitor renal functionbefore and during treatment. Use cautiously in patients on diuretics, thosewith low blood pressure, or history of urinary tract infections. Avoid inpregnancy unless necessary.
CDSCOAPPROVAL:Emphagliflozin Tablet 10/25 mg
07.05.15
FORMULATIONSAVAILABLE IN MARKET:
EMPAGLIFLOZIN 10 MG Tablets
EMPAGLIFLOZIN 25 MG Tablets
EMPAGLIFLOZIN + METFORMIN Tablets
EMPAGLIFLOZIN + LINAGLIPTIN Tablets
Note: Productprotected by valid patents are not offered for sale in countries where suchpatents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Highly Pure Medicine Grade
Empagliflozin is manufactured to a strict pharmaceutical standard with at least 99.0% purity, ensuring reliable efficacy and safety for medicinal use. Its high grade suits a wide range of pharmaceutical applications and aligns with stringent industry requirements. The low presence of impurities makes it optimal for advanced drug formulations.
Stable, Versatile Formulation
With a shelf life of up to three years when kept at room temperature (pH 7.4), this product maintains its physical and chemical integrity. The powder form and particle size ranging from 10 m to 100 m provide flexibility for various formulation processes in the pharmaceutical industry.
Ideal for Type 2 Diabetes Treatment
Empagliflozin is primarily indicated for type 2 diabetes management. Its unique mechanism targets glucose reabsorption in the kidneys, promoting glucose excretion and aiding glycemic control. This mode of action offers significant benefits for individuals striving to maintain stable blood sugar levels.
FAQs of EMPAGLIFLOZIN:
Q: How is Empagliflozin used in the treatment of type 2 diabetes?
A: Empagliflozin acts by inhibiting the reabsorption of glucose in the kidneys, which promotes the elimination of excess glucose through urine. This helps reduce blood sugar levels and is commonly prescribed by healthcare professionals as part of a comprehensive diabetes management plan.Q: What is the recommended storage condition for Empagliflozin powder?
A: Empagliflozin should be stored at room temperature to preserve its stability and potency. Avoid exposure to excessive heat, moisture, or direct sunlight to maintain its three-year shelf life.Q: When should Empagliflozin be used during diabetes therapy?
A: Empagliflozin is typically introduced when diet, exercise, and other oral medications have not achieved adequate glycemic control. It should be used under the supervision of a healthcare provider, who will determine the appropriate timing based on individual patient needs.Q: Where is Empagliflozin manufactured and supplied?
A: Empagliflozin is distributed, exported, imported, and supplied by pharmaceutical companies across India. The product is available through manufacturers, distributors, importers, suppliers, and traders in the region.Q: What are the solubility characteristics of Empagliflozin?
A: Empagliflozin is very slightly soluble in water, slightly soluble in acetonitrile and ethanol, sparingly soluble in methanol, and practically insoluble in toluene. These properties are important for formulation and manufacturing purposes.Q: What are the main benefits of using Empagliflozin?
A: The key benefit of Empagliflozin is its ability to effectively lower blood sugar levels in individuals with type 2 diabetes. Its high purity and stable shelf life also ensure consistent results and long-term storage, making it a reliable component in pharmaceutical solutions.Q: How is Empagliflozin processed for pharmaceutical use?
A: Empagliflozin is provided as a finely milled, powdery substance with a particle size range of 10 m to 100 m. It is formulated under strict quality controls to ensure minimal heavy metal content and maintained at an optimal pH of 7.4 for pharmaceutical preparations.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese