OL MESARTAN
Product Details:
- Ph Level 3-5
- EINECS No not applicable
- Molecular Weight 446.50 g/mol Grams (g)
- Solubility low solubility in water, with a mole fraction solubility of 2.29 107 at 298.15 K
- Heavy Metal (%) NA
- Particle Size 140 nm
- Molecular Formula C24H26N6O3
- Click to View more
OL MESARTAN Price And Quantity
- 1 Kilograms
OL MESARTAN Product Specifications
- OLMESARTAN
- NA
- White to Off-White Solid
- Powder
- 180-182C
- Dry Place
- C24H26N6O3
- OLMESARTAN
- Take medicine once per day by mouth. If you are using the oral liquid form, shake the container before use. Carefully measure the liquid dosage form.
- 3 Years
- Other
- 804.2 C
- 29420090
- Pharmaceutical Intermediates
- Other
- 140 nm
- 144689-24-7
- C24H26N6O3
- 99 %
- Medicine Grade
- 446.50 g/mol Grams (g)
- low solubility in water, with a mole fraction solubility of 2.29 107 at 298.15 K
- NA
- 3-5
- not applicable
OL MESARTAN Trade Information
- SAHAR AIR CARGO
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Week
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- FDCA, GMP, GLP AND ISO 9001-2014
Product Description
Niksan Pharmaceutical is one of the leading manufacturer, supplier and exporter of the Olmesartan. The best productmanufactured by Niksan Pharmaceutical is Olmesartan. We manufacture, supply and export bestquality product of Olmesartan API along with Olmesartan formulations.
Niksan Pharmaceutical provides best quality product of Olmesartanin all states of India like Kerala, Punjab, Rajasthan, Telangana, UttarPradesh, Gujarat, Delhi, Tamil Nadu, Bihar, Karnataka, Jammu and Kashmir,Maharashtra, West Bengal, Odisha, Madhya Pradesh, Andhra Pradesh, Haryana and many other states.
NiksanPharmaceutical is also manufacture and exports large quantity of Olmesartan products in whole globe since many years incountries like Guatemala,Venezuela, Honduras, Bangladesh, Nicaragua, Ireland, El Salvador, Italy, United States , Australia, Dominican Republic, Mexico, Portugal, Canada, Chile, South Korea, Spain, United ArabEmirates, Philippines and many more countries.
Olmesartan belongs to the medicine class called angiotensin receptor blockers.Olmesartan used in the treatment of hypertension. Olmesartan decrease the highblood pressure by relaxing the smooth muscle by vasodilation.
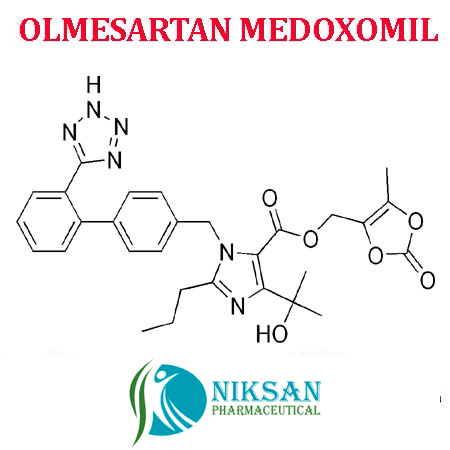
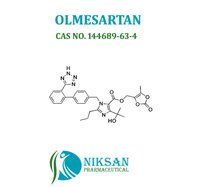
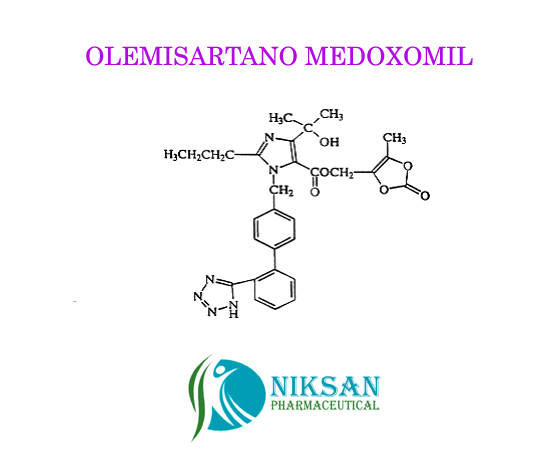
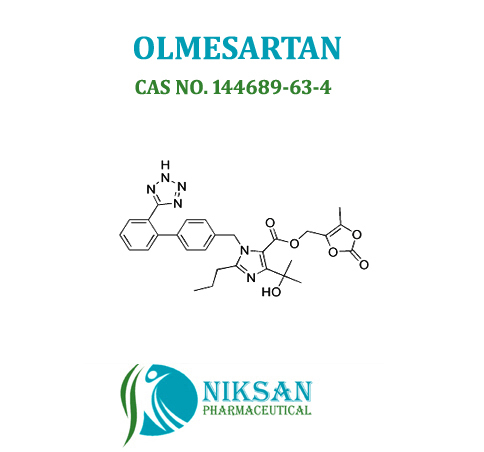
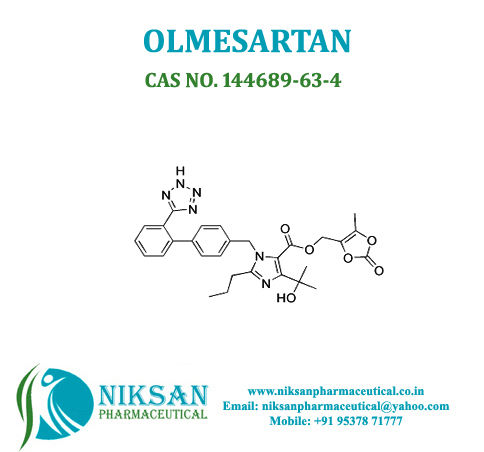
IUPAC NAME: 4-(2-hydroxypropan-2-yl)-2-propyl-1-({4-[2-(1H-1,2,3,4-tetrazol-5-yl) phenyl] phenyl} methyl)-1H-imidazole-5-carboxylic acid
CAS NO: 144689-24-7
FORMULA: C24H26N6O3
MOLECULAR MASS: 446.50 g/mol
STORAGE CONDITIONS: Store medicine in cool and dry place, awayfrom direct heat and sunlight. Do not store medicine in humid place likebathroom or kitchen. Keep away from children and pets.
HOW TO USE: Take medicine once per day by mouth. If youare using the oral liquid form, shake the container before use. Carefullymeasure the liquid dosage form.
HOW OLMESARTAN WORKS: Olmesartaninhibits the high blood pressure by binding with angiotensin II receptor.Angiotensin II cause vasoconstriction so by inhibiting the enzyme Olmesartancause vasodilation of the smooth muscle and by this blood flow increases andprevents hypertension.
PHARMACOKINETICS: Olmesartan rapidly absorbed IN GI trackafter the oral administration with the bioavaibility of 4.5% to 28.6%. Almost99% of Olmesartan binds with the blood plasma albumin. The half-life ofOlmesartan is 10-15hours. Approximately 10-16% of Olmesartan eliminated throughurine.
SIDE EFFECTS: Common side effects of Olmesartan arelightheadness and dizziness. Other side effects like fainting, high potassiumblood level, signs of kidney problem. Some other rare reactions like skin rash,itching, swelling of skin, dizziness, trouble in breathing.
PRECAUTIONS: Before talking the Olmesartan medicine,tell your doctor if you are allergic to Olmesartan. Tell your doctor about yourmedical history like liver disease, kidney disease, dehydration or anyrespiratory problem. This medicine makes you dizzy so kindly avoid takingalcohol and cannabis products. Also avoid driving or operating machineriesbecause Olmesartan makes you dizzy.
CDSCO APPROVAL: FDCof Olmesartan Medoxomil (20mg/40mg/40mg) + Hydrochlorthiazide(12.5mg/12.5mg/25mg)approved by CDSCO in India in 02.12.2005,
Amlodipine Besilate IP eq.to Amlodipine 10mg/5mg/5mg/5mg/2.5mg +Olmesartan Medoxomil 40mg/40mg/20mg/5mg/5mg + Hydrochlorothiazide IP 25mg/12.5mg/12.5mg/12.5mg/12.5mgTablet 23.11.2011
Olmesartan Medoxomil (20mg) + Amlodipine Besylate (5mg) Tabletapproved by CDSCO in India in 15.10.2007,
Metoprolol succinate ER 25mg+50mg + Olmesartan 20/20mg approved byCDSCO in India in 19.07.2010,
Olmesartan 40mg + Amlodipine 5mg (Additional Strength) approved byCDSCO in India in 09.08.2010,
Olmesartan medoximil-20mg + Ramipril-5mg. Tablet approved by CDSCOin India in 12.6.2007,
Olmesartan (20/40mg) + Atorvastatin 5/10/20mg film coated tablets approvedby CDSCO in India in 27.10.2009,
Olmesartan + Ramipril (40mg + 5mg) tablets (Additional Strength)approved by CDSCO in India in 15.09.2008,
Olmesartan (as Medoxomil) 10mg (addl. Strength) approved by CDSCOin India in 18.05.2006,
Olmesartan Medoxomil (5mg/20mg/40mg) Tablet approved by CDSCO inIndia in 21.07.2005,
Olmesartan 40mg + Hydrochlorthiazide 25mg tablet (addl. Strength)approved by CDSCO in India in 28.06.2006.
FORMULATIONS AVAILABLE IN MARKET:
Olmesartan Medoxomil 5mg tablets
Olmesartan Medoxomil20mg tablets
Olmesartan Medoxomil 40mg tablets
Olmesartan Medoxomil 10mg tablets
Olmesartan Medoxomil 20mg + amlodipine 5mg tablets
Metoprolol succinate ER 25mg+ Olmesartan 20mg tablets
Metoprolol succinate ER 50mg + Olmesartan 20mg tablets
Olmesartan + Ramipril (40mg + 5mg) tablets
Olmesartan 40mg + Hydrochlorthiazide 25mg tablets
Olmesartan 40mg + Amlodipine 5mg tablets
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Versatile Applications in Pharmaceutical Formulations
Olmesartan Medoxomil is widely used as a raw material in the manufacture of pharmaceutical tablets and capsules. Its therapeutic action targets hypertension, making it a crucial compound for cardiovascular medicines. The powder form allows for flexible formulation and manufacturing, suitable for GMP-compliant facilities.
Quality and Compliance by Niksan Pharmaceutical
Niksan Pharmaceutical has earned a reputation for excellence as a manufacturer, exporter, supplier, and distributor of Olmesartan. The product is supplied with complete documentation, including GMP certifications and ready DMF, ensuring high standards and regulatory compliance for both domestic and international markets.
FAQs of OLMESARTAN:
Q: How is Olmesartan Medoxomil utilized in pharmaceutical manufacturing?
A: Olmesartan Medoxomil serves as a key active ingredient in the production of tablets and capsules, primarily for the treatment of hypertension. Its powder form is ideal for precise blending into pharmaceutical blends and formulations.Q: What are the main benefits of using Olmesartan for hypertension?
A: Olmesartan lowers blood pressure by relaxing blood vessels through vasodilation, which helps reduce the risk of heart attacks, strokes, and other cardiovascular complications associated with high blood pressure.Q: Where should Olmesartan Medoxomil powder be stored for optimal stability?
A: It should be stored in a well-closed container in a dry place at room temperature. Proper storage conditions help maintain its potency and extend its shelf life to up to three years.Q: What documentation does Niksan Pharmaceutical provide for Olmesartan?
A: Niksan Pharmaceutical supplies Olmesartan with complete GMP certification and a ready Drug Master File (DMF), facilitating regulatory approval and compliance in global markets.Q: When should Olmesartan-based medicines be taken?
A: Medicines containing Olmesartan are typically taken once daily by mouth, as prescribed by a physician. If using the oral liquid form, shake the container prior to use and carefully measure the dosage.Q: What is the process for purchasing Olmesartan from Niksan Pharmaceutical?
A: To procure Olmesartan, contact Niksan Pharmaceutical directly as a manufacturer, supplier, and distributor. Orders can be placed for 200 kg units, customized to meet bulk requirements with supporting documentation for regulatory use.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese