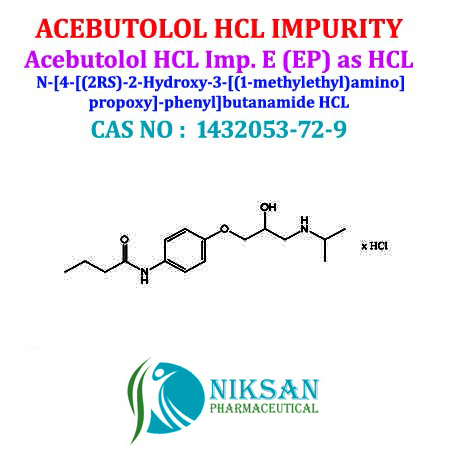

ACEBUTOLOL HCL IMPURITY E (EP)
Product Details:
- Drug Type Medicine Raw Materials
- Ingredients ACEBUTOLOL HCL IMPURITY E (EP) IS NOW A DAYS VERY NECESSORY FOR ANALYSIS BECAUSE DUE TO THE GOVERNMENT AND FOOD AND DRUG DEPARTMENT NORMS ALL THE API AND FORMULATION MANUFACTURE SHOULD BE TEST THEY MATERIAL ACCORDING TO RESPECTIVE PHARMACOPIEA AND HAVE TO HPLC ANALYSIS AS PER AS PHARMACOPIEA AND IF THERE IS SPECIFY IMPURITY MENTION IN PHARMACOPIEA THAN HAVE TO DO ANALYSIS AS PER AS PHARMACOPIEA.
- Function Other
- Dosage Guidelines ANALYSIS PURPOSE TO IDENTIFIED IMPURITY
- Suitable For Suitable For All
- Quantity 100 Unit
- Storage Instructions STORE IN WELL CLOSE CONTAINER
- Click to View more
ACEBUTOLOL HCL IMPURITY E (EP) Price And Quantity
- 10 Gram
- 1500.00 - 2000.00 INR/Gram
- 1500 INR/Gram
ACEBUTOLOL HCL IMPURITY E (EP) Product Specifications
- 100 Unit
- STORE IN WELL CLOSE CONTAINER
- Medicine Raw Materials
- Other
- Suitable For All
- ANALYSIS PURPOSE TO IDENTIFIED IMPURITY
- ACEBUTOLOL HCL IMPURITY E (EP) IS NOW A DAYS VERY NECESSORY FOR ANALYSIS BECAUSE DUE TO THE GOVERNMENT AND FOOD AND DRUG DEPARTMENT NORMS ALL THE API AND FORMULATION MANUFACTURE SHOULD BE TEST THEY MATERIAL ACCORDING TO RESPECTIVE PHARMACOPIEA AND HAVE TO HPLC ANALYSIS AS PER AS PHARMACOPIEA AND IF THERE IS SPECIFY IMPURITY MENTION IN PHARMACOPIEA THAN HAVE TO DO ANALYSIS AS PER AS PHARMACOPIEA.
ACEBUTOLOL HCL IMPURITY E (EP) Trade Information
- ANY INDIAN AIR PORT
- Letter of Credit (L/C) Western Union Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Delivery Point (DP)
- 100 Gram Per Month
- 10 Days
- Sample costs shipping and taxes has to be paid by the buyer
- ALU-ALU PACKING
- Western Europe North America Eastern Europe Middle East Central America South America Asia Africa
- Dadra and Nagar Haveli Himachal Pradesh Andaman and Nicobar Islands Uttarakhand Daman and Diu Lakshadweep South India East India Assam Arunachal Pradesh Bihar Chandigarh Delhi Gujarat Goa Haryana Jammu and Kashmir Karnataka Madhya Pradesh Maharashtra Mizoram Meghalaya Manipur Punjab Pondicherry Rajasthan Sikkim Tamil Nadu Telangana Tripura Uttar Pradesh West Bengal Nagaland North India Andhra Pradesh Kerala Central India Odisha Jharkhand West India Chhattisgarh All India
- FDCA, GMP, GLP, ISO
Product Description
NIKSANPHARMACEUTICAL IS ONE OF THE MOST TRUSTED MANUFACTURER, EXPORTER AND SUPPLIEROF ACEBUTOLOL HCL IMPURITY E (EP) N-(4-(2-HYDROXY-3-(ISOPROPYLAMINO)PROPOXY)-PHENYL) BUTYRAMIDE HYDROCHLORIDE WITH CAS NO 1432053-72-9 IT IS ALSO KNOWN AS ACEBUTOLOL HCL IMPURITY E (EP), NIKSAN PHARMACEUTICAL HAVING GMP,GLP MANUFACTURING FACILITY. WE PROVIDE ALL DOCUMENTS SUPPORT LIKE C13 NMR, H1NMR, MASS, IR, HPLC, GC, LCMS AND UV SPECTROCOY DATA OF ACEBUTOL IMPURITY.
ACEBUTOLOL HCL IMPURITY E (EP) IS ONE OF THE MOST IMPORTANT IMPURITY DOINGTESTING OF ACEUTICALOL FORMULATION OR BULK DRUGS (API). NIKSAN PHARMACEUTICAL CUSTOMIZED ALL KIND OF PHARMA ANDNON PHARMA CHEMICAL AND IMPURITY WITH SUPPORT OF HIGHLY EDUCATED AND EXPERIENCEPH D HOLDING STAFF.
NIKSANPHARMACEUTICAL MANUFACTURING, EXPORTING AND SUPPLYING 1 MG TO 100 GM OF ALLKINDL OF IMPURITY. NIKSAN PHARMACEUTICAL IS A WORLDWIDELY KNOWN EXPORTER ANDMANUFACTURER OF IMPURITY. WE ARE AMONUGST MOST FAMOUS COMPANY PROVIDINGIMPURITY WITH ALL KIND OF SUPPORTIVE DOCUMENTS.
ACEBUTOLOL HCL IMPURITY E (EP) IS NOW A DAYS VERYNECESSORY FOR ANALYSIS BECAUSE DUE TO THE GOVERNMENT AND FOOD ANDDRUG DEPARTMENT NORMS ALL THE API AND FORMULATION MANUFACTURE SHOULD BE TESTTHEY MATERIAL ACCORDING TO RESPECTIVE PHARMACOPIEA AND HAVE TO HPLC ANALYSIS ASPER AS PHARMACOPIEA AND IF THERE IS SPECIFY IMPURITY MENTION IN PHARMACOPIEATHAN HAVE TO DO ANALYSIS AS PER AS PHARMACOPIEA.
NOTE: Product projected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese






