LITHIUM CARBONATE
Product Details:
- Loss on Drying not more than 1.0%
- HS Code 28369100
- Heavy Metal (%) Max.0.002%
- Shelf Life 3 Years
- EINECS No 209-062-5
- Melting Point 723C (1333F)
- Particle Size 200 mesh
- Click to View more
LITHIUM CARBONATE Price And Quantity
- 40 Kilograms
- 5000.00 INR/Kilograms
LITHIUM CARBONATE Product Specifications
- between 9.0 and 11.0 in a 1 g/L
- Lithium carbonate is a medication used to treat manic episodes of bipolar disorder. Lithium has been used to treat manic episodes since the 19th century 3.
- 1300C
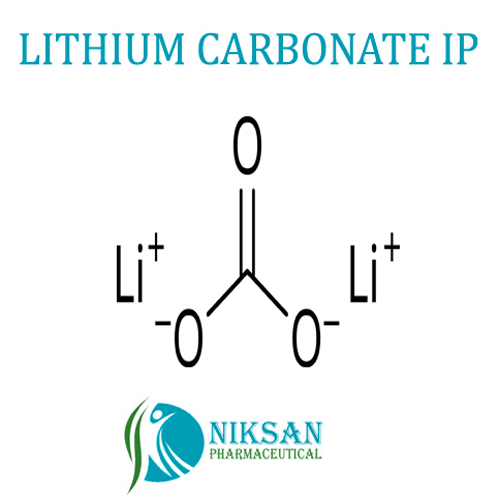
- LITHIUM CARBONATE
- Other
- Dry Place
- Slightly soluble in water, practically insoluble in ethanol (95 per cent).
- Medicine Grade
- White to Off-White Solid
- 73.891 g/mol
- 554-13-2
- Li2CO3
- 99 %
- Other
- Li2CO3
- LITHIUM CARBONATE
- Powder
- 200 mesh
- 723C (1333F)
- Max.0.002%
- 28369100
- 209-062-5
- 3 Years
- Other
- not more than 1.0%
LITHIUM CARBONATE Trade Information
- SAHAR AIR CARGO
- Cash Advance (CA), Cheque
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- FDCA, GMP, GLP and ISO
Product Description
is amedication used to treat manic episodes of bipolar disorder.
SYNONYMS: Dilithium Carbonate, Lithii Carbonas, Lithium Carbonate, Lithonate
IUPAC NAME: dilithium(1+)carbonate
CAS NO: 554-13-2
FORMULA: Li2CO3
MOLECULARMASS: 73.891 g/mol
STORAGE OF LITHIUM CARBONATE:Conditions for SafeStorage, Including Any Incompatibilities: Store in a cool, dry area. Store materialtightly sealed in properly labeled containers. Do not store together withacids. See section 10 for more information on incompatible materials.
APPLICATIONSOF LITHIUM CARBONATE:Lithium carbonate is amedication used to treat manic episodes of bipolar disorder.Lithium has been used to treat manic episodes since the 19th century 3.
HOW TO USE: Take this medication by mouth asdirected by your doctor, usually 2-3 times daily. Take lithium with or immediately after mealsto lessen stomach upset. Do not crush or chew this medication. Doing so canrelease all of the drug at once, increasing the risk of side effects.
HOW LITHIUM CARBONATE WORKS: Lithium may work by changing the release of chemicals like dopamine or serotoninin your brain.Taking lithium helps you to have more control over your emotions. It helps youcope better with bipolar mood swings.
CONTRAINDICATIONSOF LITHIUM CARBONATE: Lithium shouldgenerally not be given to patients with significant renal or cardiovascular disease, severe debilitationor dehydration, or sodium depletion, and to patients receiving diuretics, since the risk oflithium toxicity is very high in such patients.
PHARMACOKINETICSOF LITHIUM CARBONATE:Lithium carbonate is not metabolized before excretion Label.Lithium is primarily eliminated through the kidneys and elimination in thefeces is insignificant Label. The half life of lithium carbonate is18 to 36 hours Label. Other sources say it may be 7 to 20 hours 7.
SIDE EFFECTS OF LITHIUM CARBONATE: Drowsiness, dizziness, tiredness, increased thirst, increased frequency ofurination, weight gain, and mildly shaking hands (fine tremor) may occur.
PRECAUTIONS: Take care in hot weather or during activities that cause you tosweat heavily such as during hot baths, saunas, or exercise. Tell your doctorif you are pregnant or plan to become pregnant. You should not become pregnantwhile using lithium. Lithium may harm an unborn baby.
CDSCO APPROVAL:
LithiumCarbonate ER (450mg) tablet are approved by CDSCO IN 28-06-2004
FORMULATION AVAILABLE IN MARKET:
Lithiumcarbonate 250 MG Tablet
Lithiumcarbonate 300 MG Tablet
Lithiumcarbonate 400 MG Tablet
Lithiumcarbonate IP 450 MG Tablet
Lithiumcarbonate 600 MG Tablet
Note: Productprotected by valid patents are not offered for sale in countries where suchpatents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese