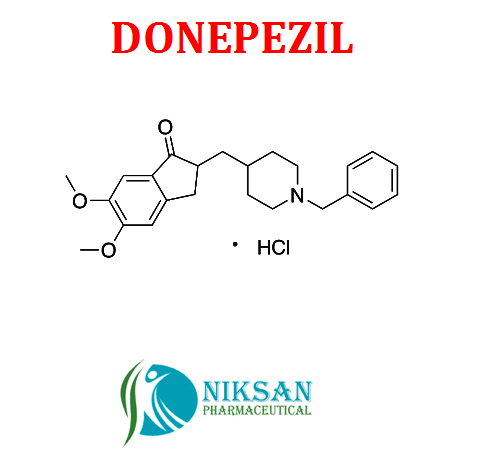
DONEPEZIL .
Product Details:
- Boiling point 527.950.0 C
- Shelf Life 3 Years
- Solubility freely soluble in chloroform, soluble in water and glacial acetic acid, slightly soluble in ethanol and acetonitrile, and practically insoluble in ethyl acetate and n-hexane
- Melting Point 225-230C
- HS Code 29420090
- Heavy Metal (%) 0.002%
- EINECS No not available
- Click to View more
DONEPEZIL . Price And Quantity
- 45000.00 INR/Kilograms
- 1 Kilograms
DONEPEZIL . Product Specifications
- freely soluble in chloroform, soluble in water and glacial acetic acid, slightly soluble in ethanol and acetonitrile, and practically insoluble in ethyl acetate and n-hexane
- Medicine Grade
- 225-230C
- 99 %
- 0.002%
- 29420090
- DONEPEZIL
- 3 Years
- 527.950.0 C
- C24H29NO3
- C24H29NO3
- 2-((1-Benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one hydrochloride
- 120014-06-4
- not available
- Room Temperature
- 379.49 g/mol Grams (g)
- 87.20.11 nm
- 1.2 to 6.8
- White to Off-White Solid
- Other
- Powder
- Donepezil is the medication used to treat the Alzheimer disease.Alzheimer disease is the condition which causes brain cells to die or waste away and by this Alzheimers disease can cause mental disorders and loss of memory.Donepezil belongs to the cholinesterase inhibitors class of medicine.
- 105C
- Other
- Pharmaceutical Intermediates
DONEPEZIL . Trade Information
- SAHAR AIR CARGO
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Dadra and Nagar Haveli, Chandigarh, Jammu and Kashmir, Andaman and Nicobar Islands, Uttarakhand, Daman and Diu, Pondicherry, Nagaland, East India, Arunachal Pradesh, Tamil Nadu, Lakshadweep, Madhya Pradesh, Goa, Rajasthan, Delhi, Central India, North India, West India, Odisha, Karnataka, Telangana, Jharkhand, Haryana, Andhra Pradesh, Himachal Pradesh, Manipur, Tripura, Punjab, West Bengal, South India, Meghalaya, Sikkim, Mizoram, Assam, Uttar Pradesh, Maharashtra, Gujarat, Bihar, Kerala, Chhattisgarh, All India
- FDCA, GMP, GLP AND ISO
Product Description
is words top leading manufacturer, exporter, trader and supplier of DonepezilAPI as well as finished pharmaceutical products of Donepezilamong the pharmaceutical companies. Our product Donepezilis widely used and appreciated by our group companies and also ourcustomers and users all around the nations. We offer the Donepezilin very affordable price.
NiksanPharmaceutical provides API and finished formulations of Donepezil in all over Indian states Like Kerala, Gujarat, Haryana,Rajasthan, Madhya Pradesh, Uttar Pradesh, Rajasthan, Karnataka, Meghalaya,Tamilnadu, Goa, Sikkim, Assam, Punjab, Delhi, Bihar, Jammu Kashmir Etc.
Niksan Pharmaceuticalis also large exporters of the API and finished pharmaceuticalproducts of Donepezil in manycountries for years. The countries where we exporting are Puerto Rico, UnitedStates, Finland, Ireland, New Zealand, Canada, United Kingdom, Belgium, Sweden,Australia , South Korea Austria, Hong Kong, Singapore, Germany, Philippines, Israel,Italy, Romania, Nigeria, Czechia, Denmark, Switzerland, Norway , Croatia,Greece, Taiwan, Malaysia, United Arab Emirates , Portugal, Poland, Thailand,Indonesia, Egypt , Iran, Hungary, Brazil, Saudi Arabia, Pakistan , SouthAfrica, France, Turkey, China, Vietnam , Netherlands, Mexico, Colombia, Spain,Japan and many more countries.
Donepezil is the medication used totreat the Alzheimer disease. Alzheimer disease is the condition which causesbrain cells to die or waste away and by this Alzheimers diseasecan cause mental disorders and loss of memory. Donepezil belongs to the cholinesterase inhibitorsclass of medicine.
SYNONYMS: Domepezil, Donepezil, Donepezilo,Donepezilum.
IUPAC NAME OF DONEPEZIL:
CAS NO: 120014-06-4
FORMULA: C24H29NO3
MOLECULAR MASS: 379.49 g/mol
STORAGE CONDITIONS: Store in cool and dry place away from themoisture and sun light. Do not place it in bathroom or humid place. Keep awayfrom children and pets.
HOW TO USE DONEPEZIL: Do not crush, chew or spit the medication.
HOW DONEPEZILWORKS: Donepezilused in the Alzheimer disease but Donepezil does not cure the disease it isonly improve the memory, awareness, and treat confusion. Donepezil inhibits theacetyl cholinesterase enzyme activity and by this process improves the mentalcondition and brain work.
PHARMACOKINETICS OF DONEPEZIL: Donepezil absorbed slowly by the GI trackafter the oral administration. Donepezil have 100% bioavaibility and reaches Tmax in 3-4 hours. Almost 96% of Donepezil binds with the plasmaprotein in the body. The half-life of Donepezil is very high. Donepezil hashalf-life of 70 hours. Almost 57% of Donepezil eliminated through the urinationand only 5% Donepezil eliminated in feces.
SIDE EFFECTS: The common side effects like nausea,vomiting, tiredness, shakiness, muscle cramps, weakness, trouble in sleeping,dizziness are seen in the patients. Tell your doctor if you have effects likeblack stool, vomit that look like coffee, stomach or abdominal pain, trouble inurination. The allergic reaction is very rare like rash, itching, swelling oftongue, face, trouble in breathing.
PRECAUTIONS: Tell your doctor your medical history if youhave intestinal disease like bleeding, trouble in urination, asthma, COPD<fainting, stomach disease etc. Do not take medicine if you are pregnant or inlactation period. This drug has dizziness effect so do not consume alcohol ormarijuana while completing the dose. Donepezil cause heart problems so kindlytake your doctors advice.
CDSCO APPROVAL: Donepezil (for additional indication)approved by CDSCO in India in 10.08.2005, Donepezil HCl. approved by CDSCO inIndia in 22.03.2001,
Donepezil Hydrochloride Orodispersible Tablet 5mg/10mg approved byCDSCO in India in 15.10.2009,
Memantine 5mg /10mg + Donepezil 5mg Tablets approved by CDSCO inIndia in 31.01.2008,
Donepezil Sustained Release Tablet 23mg (Modified Dosage Form)03.06.2011.
FORMULATION AVAILABLE IN MARKET:
Donepezil 5mg tablets
Donepezil 10mg tablets
Donepezil 23mg tablets
Donepezil 5mg + Memantine 5mg tablets
Donepezil 5mg + Memantine 10mg tablets
Donepezil Hydrochloride Orodispersible Tablets 5mg
Donepezil Hydrochloride Orodispersible Tablets10mg
Donepezil HCl tablet
Note: Product protected by valid patents are notoffered for sale in countries where such patents are still valid and itsliability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Efficient Manufacturing & Quality Assurance
Niksan Pharmaceutical brings years of expertise as a manufacturer, exporter, supplier, and distributor of Donepezil. Operations adhere strictly to GMP guidelines, ensuring that every batch meets rigorous standards. Ready DMF documentation streamlines international regulatory compliance, making global distribution secure and straightforward.
Proper Usage & Dosage Recommendations
Donepezil is typically formulated into tablets or capsules for oral administration to treat Alzheimers disease. Patients should take the medication with or without food but must avoid crushing, chewing, or spitting the tablets or capsules. Adhering to these guidelines ensures optimal effectiveness of the medication.
Storage & Stability
To preserve its potency, Donepezil should be stored in a well-closed container in a cool, dry environment, away from direct sunlight and moisture. It is important not to keep it in bathrooms or other humid places. Under recommended conditions, Donepezil maintains its efficacy for up to three years.
FAQs of DONEPEZIL:
Q: How is Donepezil used in the treatment of Alzheimers disease?
A: Donepezil acts as a cholinesterase inhibitor, enhancing brain neurotransmitter levels to help manage symptoms of Alzheimers disease such as memory loss and cognitive decline. It is prescribed in tablet or capsule form for oral administration.Q: What is the recommended process for taking Donepezil tablets or capsules?
A: Donepezil should be taken by mouth, with or without food, as directed by a healthcare professional. Tablets and capsules must be swallowed whole; do not crush, chew, or spit them to ensure proper absorption and effectiveness.Q: Where should Donepezil powder be stored to maintain its stability?
A: Store Donepezil in a well-closed container, in a cool and dry place, protected from moisture and direct sunlight. Avoid areas like bathrooms or humid environments to prevent degradation of its quality.Q: What are the benefits of choosing Niksan Pharmaceuticals Donepezil?
A: Niksan Pharmaceutical offers high-purity Donepezil produced in GMP-certified facilities with a ready DMF, ensuring product reliability, compliance, and consistent supply for global partners.Q: When is the products shelf life valid, and how long does Donepezil remain effective?
A: Donepezil remains effective for up to three years from its manufacturing date when stored according to recommended guidelines, ensuring long-term usability for pharmaceutical production.Q: How can Donepezil be sourced in bulk for pharmaceutical manufacturing?
A: Bulk quantities of Donepezil (100 kg units) can be sourced directly from Niksan Pharmaceutical, a reliable manufacturer, exporter, supplier, and distributor with global reach and industry certifications.Q: What are the physical and chemical properties of Donepezil?
A: Donepezil is a white to off-white solid powder with a molecular weight of 379.49 g/mol. It is freely soluble in chloroform and water, slightly soluble in ethanol and acetonitrile, and displays stability in dry, cool storage.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese