CLOBETASOL PROPIONATE
Product Details:
- Loss on Drying 0.5% to 2.0%
- HS Code 29420090
- Heavy Metal (%) NA
- Taste Other
- EINECS No 246-634-3
- Melting Point 196C
- Molecular Weight 466.97 g/mol Grams (g)
- Click to View more
CLOBETASOL PROPIONATE Price And Quantity
- 65000 INR/Kilograms
- 5 Kilograms
CLOBETASOL PROPIONATE Product Specifications
- CLOBETASOLE PROPIONATE
- 196C
- 466.97 g/mol Grams (g)
- 246-634-3
- 25122-46-7
- Other
- NA
- 29420090
- 0.5% to 2.0%
- Medicine Grade
- Powder
- 99 %
- very low solubility in water, but is soluble in some organic solvents
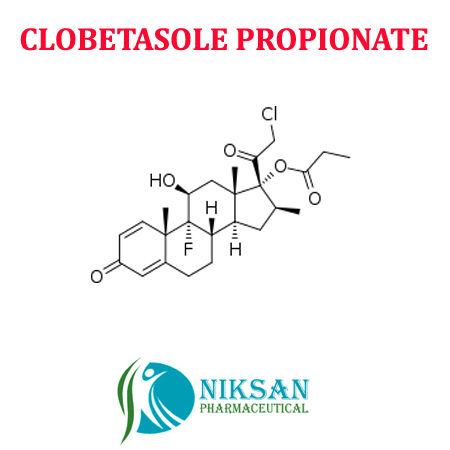
- C25H32ClFO5
- Room Temperature
- White to Off-White Solid
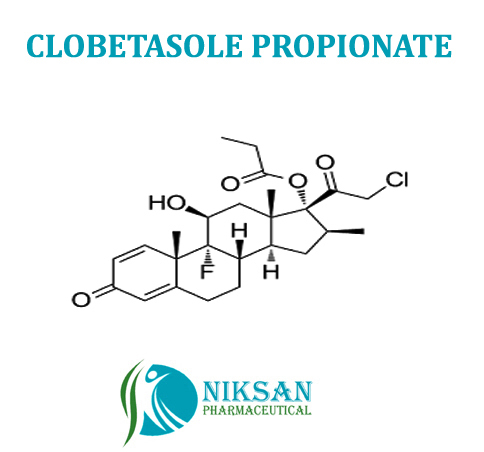
- (11,16)-21-Chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)pregna-1,4-diene-3,20-dione
- C25H32ClFO5
- 569C
- 5.5
- Other
- Clobetasol Propionate is class I corticosteroid medication. Clobetasol Propionate used in the treatment of many skin disease like skin rash, eczema, seborrheic dermatitis, psoriasis, contact dermatitis. Clobetasol Propionate is used in skin infections. Comes in many dosage forms like shampoo, cream, lotions, and ointment
- Other
- 3 Years
- 50 m or less
CLOBETASOL PROPIONATE Trade Information
- NHAVA SHEVA
- Cash Advance (CA), Cheque
- 100 Kilograms Per Week
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Meghalaya, North India, Himachal Pradesh, Chhattisgarh, Arunachal Pradesh, Chandigarh, Jammu and Kashmir, West Bengal, Dadra and Nagar Haveli, Kerala, Punjab, Bihar, Telangana, Lakshadweep, Pondicherry, Maharashtra, Uttarakhand, Sikkim, Tripura, Mizoram, Central India, Rajasthan, Odisha, South India, Assam, Goa, Gujarat, Andaman and Nicobar Islands, Jharkhand, Madhya Pradesh, Haryana, Manipur, Delhi, Andhra Pradesh, Uttar Pradesh, Daman and Diu, West India, Nagaland, Karnataka, East India, Tamil Nadu, All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceuticalis one of the leading Manufacturer, Exporter, Distributor, andSupplier ofClobetasol Propionatein Ankleshwar, Gujarat, India. To understand the largestaccomplishment of clients, we present these products at very realistic priceranges. Niksan Pharmaceutical and Niksan group companies are now a days one ofthe largest manufacturer and exporter of Clobetasol Propionate API and alsofinished formulations.
Niksan PharmaceuticalprovidesClobetasol Propionate to all states of India like Punjab, Rajasthan, Andhra Pradesh, Haryana,Telangana, Bihar, Karnataka, Delhi, Tamil Nadu, Odisha, Maharashtra, West Bengal,Uttar Pradesh, Gujarat, and Madhya Pradesh etc. We also give gift samples tothe companies for R&D purpose.
Niksan Pharmaceuticalis one of leading manufacturer and exporter of Clobetasol Propionate for manyyears in all the countries like Puerto Rico, United States, Nepal, Philippines,Hong Kong, Nigeria, Bangladesh, United Arab Emirates, Pakistan, Taiwan,Singapore, Malaysia, Canada, Indonesia, Thailand, New Zealand, Vietnam, Israel,United Kingdome, Saudi Arabia, Chile, Australia, Colombia, France, Germany,Brazil and many other countries.
Clobetasol Propionateis class I corticosteroid medication. Clobetasol Propionate used in thetreatment of many skin diseases like skin rash, eczema, seborrheic dermatitis,psoriasis, contact dermatitis. Clobetasol Propionate is used in skininfections. Comes in many dosage forms like shampoo,cream, lotions, andointment
SYNONYMS:Clobetasol 17-propanoate, Clobetasol 17-propionate, Clobetasolpropionate, Clobetasol propionate E
IUPAC NAME:(1R,2S,10S,11S,13S,14R,15S,17S)-14-(2-chloroacetyl)-1-fluoro-17-hydroxy-2,13,15-trimethyl-5-oxotetracycloheptadeca-3,6-dien-14-ylpropionate
CAS NO:25122-46-7FORMULA:C25H32ClFO5
MOLECULAR MASS:466.97g/mol
STORAGE CONDITIONS:Store in cool and dry place, away from light and direct heat. Do notplace medicine in bathroom or humid place. Keep away from children and pets.
HOW TO USE:Do not useClobetasol Propionate on face, under arms or onopen wounds. Wash and clean the affected area and after that apply a thin filmof Clobetasol Propionate gel on the area and gently rub it. Two time a day.
HOW CLOBETASOL PROPIONATEWORKS:Clobetasol Propionate bindswith the glucocorticoid receptor and inhibits the pro-inflammatory receptor.Clobetasol Propionate promotes the anti-inflammatory signals to the brain andgives inflammatory effects
PHARMACOKINETICS:Clobetasol Propionate absorbed systemic circulation after theapplication. Clobetasol Propionate takes 5 hours to reach the peak plasmaconcentration after using 2 times daily. The bioavailability of ClobetasolPropionate is only 2.09%. The half-life of Clobetasol Propionate is 96hours.The medicine metabolise in liver and after it will excrete by biliary rout.
SIDE EFFECTS:Normally burning, skin irritation, stinging, dry skin, redness myseen at the site where medicine was applied. Contact your doctor If you seesome side effects like stretch marks; skin tightening, acne, skin thinning, andunwanted hair grow. Some rare side effects like weakness, fainting, skinburning, rash, itching, weight loss, headache seen in patients.
PRECAUTIONS:Tell your doctor if you are allergic to the medication. If you havemedical history like weak immune system, skin problem, poor blood circulationproblem, liver problem tell your doctor before using the medication. The medicationcan temporarily slow down the child growth so take advice from doctor beforegiving to children. Do not use this medication if you are in pregnancy or inlactation period.
CDSCO APPROVAL:Clobetasol propionate + Ammonium lactategel is approved by CDSCO inIndia in 07.08.1996
Clobetasolpropionatecream/ointment approved by CDSCO in India in October-1987
Clobetasol propionate bulkapproved by CDSCO in India in October-1987
Nadifloxacin (1%) +Clobetasol (0.05%) approved by CDSCO in India in 06.10.2003
FORMULATIONS AVAILABLE INMARKET:
Clobetasol propionate 0.05%w/w cream
Clobetasol propionate 0.05%w/v solution
Clobetasol propionate0.025% w/w gel
Clobetasol propionate0.05%w/w + Clotrimazole 1%w/w cream
Clobetasol propionate 0.05%w/w + Miconazole 2%w/w cream
Clobetasol propionate0.05%w/w + Luliconazole 1% w/w cream
Clobetasol propionate0.05%w/w + Terbinafine 1% w/w cream
Clobetasol propionate0.05%w/w + Nadifloxacin 1% w//w cream
Clobetasol propionate0.05%w/w + Salicylic acid 3% w/w cream
Clobetasol propionate0.05%w/w+ Fusidic acid 2% w/w cream
Clobetasol propionate 0.05%w/v+ Salicylic acid 3%w/v gel
Note:Product protected by valid patents are not offered for sale incountries where such patents are still valid and its liability is at BuyersRisk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Advanced Treatment for Skin Conditions
Clobetasol Propionate stands out as a class I corticosteroid, recognized for its efficacy in treating severe dermatological issues. Suitable for addressing symptoms of eczema, psoriasis, and a range of dermatitis types, this potent powder works to swiftly alleviate inflammation, itching, and redness. It is typically offered as a raw material for further compounding into creams, ointments, lotions, or shampoos.
Quality and Handling Specifications
Manufactured to stringent quality standards, Clobetasol Propionate features 99% purity, fine particle size (50 m or less), and a stable shelf life of three years. The product should be securely stored at room temperature in a cool, dry environment, away from direct light or humidity, preserving both its potency and stability for pharmaceutical applications.
FAQs of CLOBETASOL PROPIONATE:
Q: How should Clobetasol Propionate powder be stored?
A: Clobetasol Propionate should be kept in a cool, dry place, away from light and direct heat. Avoid storing it in humid areas such as bathrooms to preserve its effectiveness over time.Q: What is the primary use of Clobetasol Propionate?
A: This ingredient is recommended for the treatment of various skin conditions, including eczema, contact dermatitis, seborrheic dermatitis, psoriasis, and certain skin infections, due to its strong anti-inflammatory and immunosuppressive effects.Q: When should Clobetasol Propionate be applied to the skin?
A: It is best applied after cleaning and drying the affected area. Gently spread a thin film over the skin and rub in as directed by your healthcare provider, typically once or twice daily depending on severity and prescription.Q: Where can Clobetasol Propionate be used?
A: Clobetasol Propionate can be applied to affected external skin regions as directed by a physician but should be avoided on the face, groin, or areas with broken skin unless recommended by a doctor.Q: What is the process for using Clobetasol Propionate in formulations?
A: As a raw material, Clobetasol Propionate can be compounded by pharmacists or manufacturers into various dosage forms such as creams, ointments, shampoos, or lotions, depending on therapeutic needs.Q: What are the benefits of choosing high-purity Clobetasol Propionate?
A: With a 99% purity level and controlled particle size, this product ensures reliable efficacy and consistency when formulated into dermatological medicines, supporting optimal patient outcomes.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese