AR TEMETHER
Product Details:
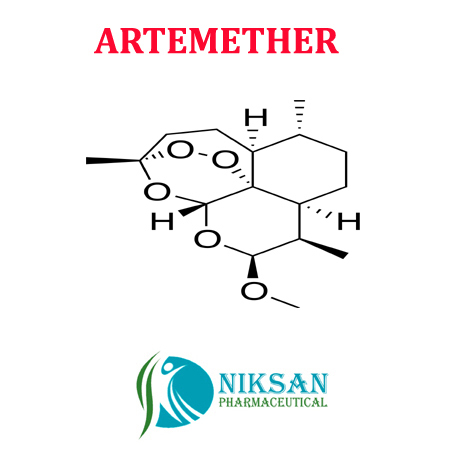
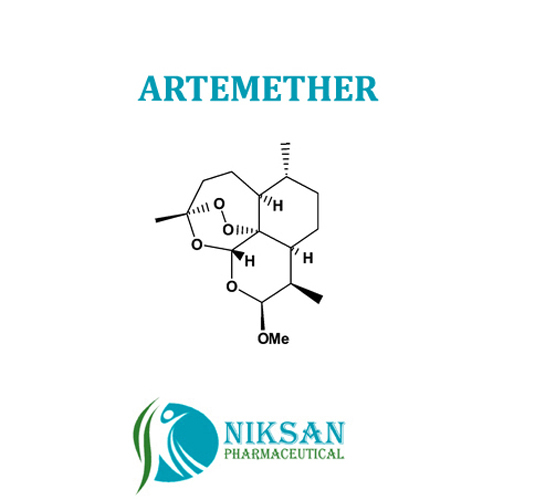
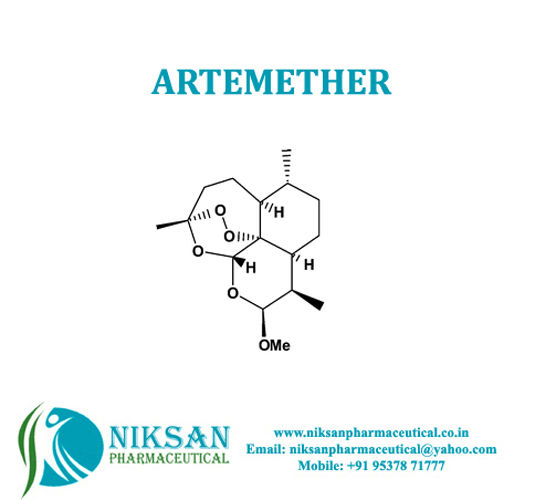
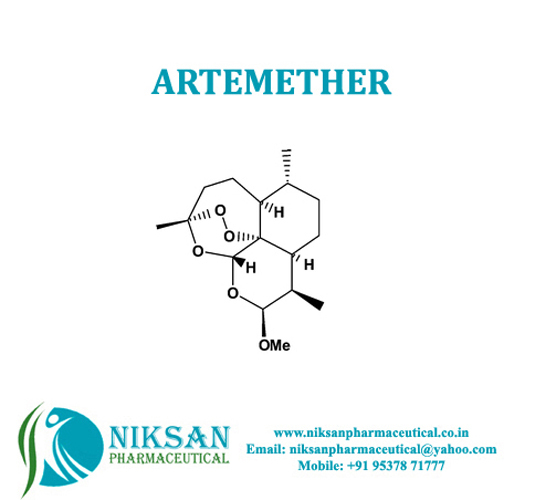
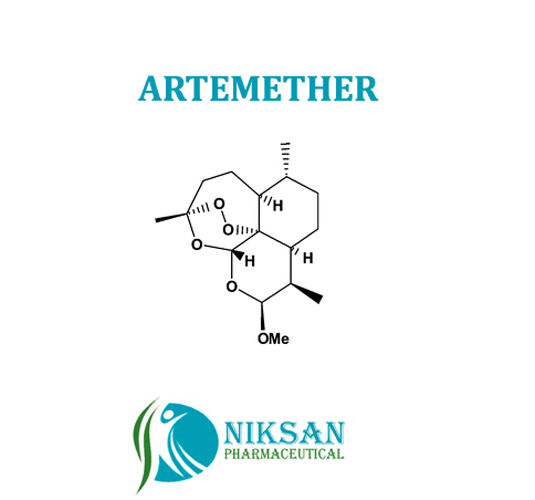
- Molecular Formula C16H26O5
- Storage Room Temperature
- HS Code 29420090
- Shelf Life 3 Years
- Smell Other
- Boiling point NA
- Solubility low aqueous solubility
- Click to View more
AR TEMETHER Price And Quantity
- 5 Kilograms
- 23000.00 INR/Kilograms
AR TEMETHER Product Specifications
- low aqueous solubility
- Other
- 3 Years
- 29420090
- NA
- White to Off-White Solid
- Other
- Medicine Grade
- 99 %
- Other
- Room Temperature
- C16H26O5
- 71963-77-4
- 86 to 90 C
- Artemether belongs to the antimalarial class of medicine. Artemether used in the treatment of malaria caused by the plasmodium parasites. Artemether prevents the malaria by killing the parasite that causes the disease. Artemether give intra muscular injection.
- Artemether
- C16H26O5
- nanometer range
- 3.0 to 10.0
- less than 0.5%
- less than 0.002%
- 298.37 g/mol GSM (gm/2)
- 71963-77-4
- ARTEMETHER
- Powder
AR TEMETHER Trade Information
- NHAVA SHEVA
- 100 Kilograms Per Month
- 1 Days
- No
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
- WHO,GMP,GLP,ISO
Product Description
The Niksan Pharmaceutical is among world top most manufacturer, supplier, exporter and trader of the Artemether finishedformulations moreover Artemether API. NiksanPharmaceutical is the huge manufacturer, exporter and supplier of Artemether.API and formulation situated in Ankleshwar, Gujarat, India.
NiksanPharmaceutical provides API andfinished formulations of Artemether in all over Indian States Like Kerala,Gujarat, Haryana, Rajasthan, Madhya Pradesh, Uttar Pradesh, Rajasthan,Karnataka, Meghalaya, Tamil nadu, Goa, Sikkim, Assam, Punjab, Delhi, Bihar,Jammu Kashmir Etc.
NiksanPharmaceutical is alsolarge exporter of the API and finished pharmaceutical products of Artemether inmany countries for years. The countries where we exporting are Cameroon, Niger,Mali, Somalia, Ghana, Malawi, Cite devoir, Burkina Faso, Nigeria, Tanzania,Angola, Sudan, Congo Kinshasa, Uganda, Kenya, Zambia, Pakistan, Yemen, SouthAfrica, Bolivia, Saudi Arabia, Colombia, Philippines, Australia, Netherlands,United Kingdom, France, Germany, United States, Canada, Brazil and many morecountries.
Artemether belongs to the antimalarial class ofmedicine. Artemether used in the treatment of malaria caused by the plasmodiumparasites. Artemether prevents the malaria by killing the parasite that causesthe disease. Artemether give intra muscular injection.
SYNONYMS: Altimeter,Artemether, Artemetherum, Artemisininelactol methyl ether, Dihydroartemisininmethyl ether, Dihydroqinghaosu methyl ether, methyl-dihydroartemisinine
IUPAC NAME:(1R,4S,5R,8S,9R,10S,12R,13R)-10-methoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclohexadecane
CASNO: 71963-77-4
FORMULA: C16H26O5
MOLECULAR MASS: 298.37 g/mol
STORAGE CONDITION: Store in cool and dry place, away from direct heat and sun light.
Do not placein bathroom orkitchen. Keep away from children and pets.
HOW TO USE: Take this medication with or without food as prescribed by your doctor.
Take this medication twicea day for 3 days. Take medication with food or milk to prevent the stomach upset.Take Artemether injection intra muscularly. Take the medication regularly toget more benefits.
HOW ARTEMETHER WORKS: Artemetheris peroxide containing anti-malarial medicine. Artemethercause degradation of haemoglobin by product and inhibits the O2 carryingcapacity in the parasite. Thus Artemether act as anti-malarial medicine bykilling and preventing the growth of plasmodium parasite.
PHARMACOKINETICS: Artemether absorbed in body after the oral and I.M.administration. The absorption of Artemether increases when taken with food.Almost 47%-76% of Artemether binds with the blood plasmaprotein. The half-lieof the medicine is between 1.9-2.2 hours. The Artemether eliminated through thehepatic pathway.
SIDE EFFECTS: The normal side effects like headache, sweating, nausea, vomiting,insomnia, stomach pain, cough, fever, chills, and dizziness. Contact yourdoctor if you see side effects like chest pain, dizziness, and fainting,irregular heartbeats. There are some rare allergic reactions like rash,swelling, itching.
PRECAUTIONS: Tell your doctor if you have any allergic reactions to Artemether.
Tell your doctor if youhave any conditions like heart problem, stomach problem, liver problem,abdominal problem, kidney problem. Artemether causes heart related problem sokindly tell your doctor before using the Artemether medication.
CDSCO APPROVAL: Artemether + Lumefantrineapproved by CDSCO in India in 26.12.2002,
Artemether40mg/80mg +Lumefantrine 240mg/480mg tablets (additional Strength) approved byCDSCO inIndia in24.06.2008,
Artemether +Lumefantrine Dispersible tablet(40/80mg + 240/480mg) (Additional Strength)approved by CDSCO in India in 05.08.2008,
Artemether + Lumefantrine(80mg + 480mg/5ml)Dry syrup approved by CDSCO in India in 30.09.2008,
Artemether injectionapproved by CDSCO inIndia in02.08.1996
FORMULATIONS AVAILABLE INMARKET:
Artemether 40 MG tablets
Artemether 80 MG /1MLsolution
Artemether 150 MG tablets
Artemether 80MG+Lumefantrine 480 MG tablets
Artemether 20MG+Lumefantrine 120 MG /ML solution
Artemether 20MG+Lumefantrine 120 MG tablets
Artemether 40MG+Lumefantrine 240 MG tablets
Artemether 80MG+Lumefantrine 840 MG tablets
Artemether 60MG+Lumefantrine 360 MG tablets
Artemether 40MG+Lumefantrine 240 MG /5 ML solutions
Note: Product protected by valid patents are not offered for sale incountries where such patents are still valid and its liability is at BuyersRisk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese