VOGLIBOSE
Product Details:
- Molecular Formula C10H21NO7
- Storage Dry Place
- Molecular Weight 267.28 g/mol Grams (g)
- Shelf Life 3 Years
- HS Code 29420090
- Medicine Name VOGLIBOSE
- Chemical Name VOGLIBOSE
- Click to View more
VOGLIBOSE Price And Quantity
- 1 Kilograms
VOGLIBOSE Product Specifications
- 3 Years
- Voglibose is belongs to drugs class alphaglucosidase inhibitorwhich used for lowering postprandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macro vascular complications. Voglibose is used for treatment of type II Diabetes Mellitus also used in control blood sugar level in type II Diabetes.
- White to Off-White Solid
- VOGLIBOSE
- 29420090
- Powder
- C10H21NO7
- Dry Place
- VOGLIBOSE
- 267.28 g/mol Grams (g)
- 83480-29-9
- 99 %
- Medicine Grade
- Other
VOGLIBOSE Trade Information
- SAHAR AIR CARGO
- Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C) Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINER
- Western Europe Eastern Europe Middle East Africa South America Australia Asia Central America North America
- Andaman and Nicobar Islands Chandigarh Tripura Nagaland Daman and Diu Dadra and Nagar Haveli Lakshadweep South India East India Assam Arunachal Pradesh Bihar Delhi Gujarat Goa Jammu and Kashmir Jharkhand Karnataka Madhya Pradesh Maharashtra Mizoram Meghalaya Manipur Odisha Punjab Pondicherry Rajasthan Sikkim Tamil Nadu Uttarakhand West Bengal All India Uttar Pradesh Haryana North India Telangana Andhra Pradesh Kerala Central India West India Chhattisgarh Himachal Pradesh
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical is leading manufacturer and distributor of the Voglibose among the other countries. Niksan Pharmaceutical is one of the major the supplier, distributor, exporter and manufacturer of the Voglibose API and finished formulations in Ankleshwar, Gujarat, India. The products of Niksan Pharmaceutical and Niksan group companies are widely appreciated by the clients and other companies.
Niksan Pharmaceutical provides API and finished formulations of Voglibose in many Indian states of like Uttarakhand, AndhraPradesh, Kerala, Tamil Nadu, Odisha, Jharkhand, Telangana, Himachal Pradesh, Karnataka, West Bengal,Gujarat, Maharashtra, Delhi, Haryana, Jammu & Kashmir, Punjab, UttarPradesh, Assam, Chandigarh, Madhya Pradesh, Bihar, Rajasthan Etc.
Niksan Pharmaceutical is also huge exporter of the API and finished pharmaceutical products of Voglibose in many countries for years. The countries where we exporting are Japan,United Arab Emirates, Ukraine, Bolivia, Bangladesh, Philippines, Thailand,Saudi Arabia, Italy, Australia, Canada, United States, United Kingdom, Mexico,Indonesia and many other countries.
Voglibose is belongs to drugs class alpha glucosidase inhibitor which used for lowering postprandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macro vascular complications.
Voglibose is used for treatment of type II Diabetes Mellitus also used in control blood sugar level in type II Diabetes.
SYNONYMS: Voglibosa,Voglibose, Voglibosum
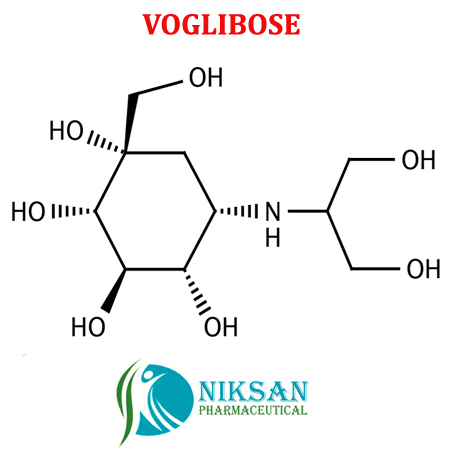
IUPAC NAME: (1S,2S,3R,4S,5S)-5-[(1,3-dihydroxypropan-2-yl)amino]-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol.
CAS NO: 83480-29-9
FORMULA: C10H21NO7
MOLECULAR MASS:267.28 g/mol
STORAGE OF VOGLIBOSE: Store it in cool and dry place, away from moisture and direct light. Keep medicine away from the reach of children and pets. Does not store medicine in bathroom or any humidly place.
APPLICATIONS OF VOGLIBOSE: Voglibose is used to improve blood sugar control in adults with type IIdiabetes. It is used in the treatment of type IIdiabetes mellitus. Voglibose is an anti-diabetic medicine. It inhibits the intestinal enzymes responsible for breaking complex sugars like glucose.
HOW TO USE: Voglibose should taken by mouth with or without food.
Follow all directions on your prescription label or as your doctor recommends you.
HOW VOGLIBOSE WORKS: Absorption of glucose delays by Voglibose and also Voglibose cause risk reducing macro vascular complications.
CONTRAINDICATIONS: Do not take Voglibose if you have Diabetic ketoacidosis. Avoid the Voglibose in inflammatory bowed disease, colonic ulcerations and partial intestinal obstructions. Kindly share the information with doctor if you have any chronic disorders of digestion or absorption. Take your doctor advice if you are suffering of Hypersensitivity to the drug or to any of its components.
PHARMACOKINETICS OF VOGLIBOSE: Absorption is weakly if you take Voglibose by oral administration.Half life of Voglibose is 4.0 8 hours. Metabolism of Voglibose is minor in liver. The Voglibose excretion is minor and plasma concentrations after oral dose have been undetectable.
SIDE EFFECTS OF VOGLIBOSE: The common side effects of Voglibose are diarrhea,flatulence, Pneumatises Intestinalis, Abnormal liver function, Nausea or vomiting and Dizziness.
PRECAUTION: Inform the doctor if the patient is allergic to the active ingredients and any other drugs like Laparotomy or ileus, Stenos is, Shortness of breath, Severe hepatic Etc.
Do not take Voglibose if you have Roemheld's syndrome.
CDSCO APPROVAL:Voglibose0.2 mg + Metformin 500mg Tablets are approved by CDSCO in India in 13.07.2009.
Voglibose disp.tab .0.2mg/0.3mg tablets are approved by CDSCO in India in 20.11.2007.
Voglibose 0.3 + Metformin (SR)500mg tablets are approved by CDSCO in India in 06.08.2010.
Voglibose 0.2mg/0.3mg tablet(Mouth dissolving) is approved by CDSCO in India in 28.12.2007.
Voglibose (0.2mg/0.3mg) Tablets are approved by CDSCO in India 17.11.2005.
FORMULATIONS AVAILABLE IN MARKET:
Voglibose 0.3 MG Pellets
Voglibose 0.2 MG Pellets
Voglibose 0.2 mg + Metformin 500mg Tablets
Voglibose disp. tablets 0.2mg/0.3mg
Voglibose 0.3 + Metformin (SR)500mg tablets
Voglibose 0.2mg/0.3mg tablets(Mouth dissolving)
Voglibose (0.2mg/0.3mg) Tablets
Glimepiride 2 MG+Metformin 500MG+Voglibose 0.2 MG tablets
Glimepiride 1 MG+Metformin 500MG+Voglibose 0.2 MG tablets
Glimepiride 1 MG+Metformin 500MG+Voglibose 0.3 MG tablets
Repaglinide 0.5 MG+Voglibose 0.3MG tablets
Repaglinide 1 MG+Voglibose 0.2 MG tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese