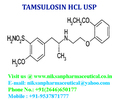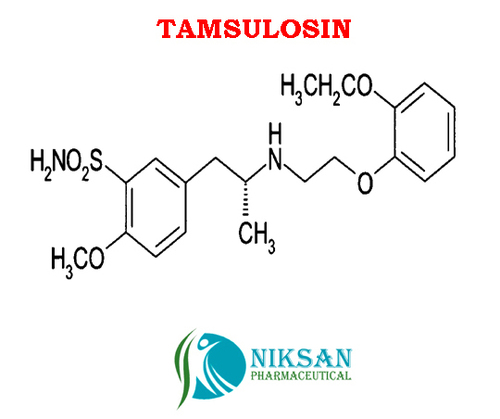
TAMSULOSIN
Product Details:
- Loss on Drying 0.5 %
- Heavy Metal (%) 0.5 %
- Taste Odorless
- Particle Size 10 MICRON
- Shelf Life 3 Years
- Molecular Weight 408.51 Grams (g)
- HS Code 29420090
- Click to View more
TAMSULOSIN Price And Quantity
- 1 Kilograms
TAMSULOSIN Product Specifications
- White crystalline powder
- 0.5 %
- 0.5 %
- Powder
- 106133-20-4
- Other
- 3 Years
- TAMSULOSIN
- Odorless
- 10 MICRON
- Tamsulosin is a medication used to treat symptomatic benign prostatic hyperplasia (BPH), help with the passage of kidney stones,[2] and for urinary retention along with other measures.
- Medicine Grade
- 99.8 %
- 408.51 Grams (g)
- Resinous
- 29420090
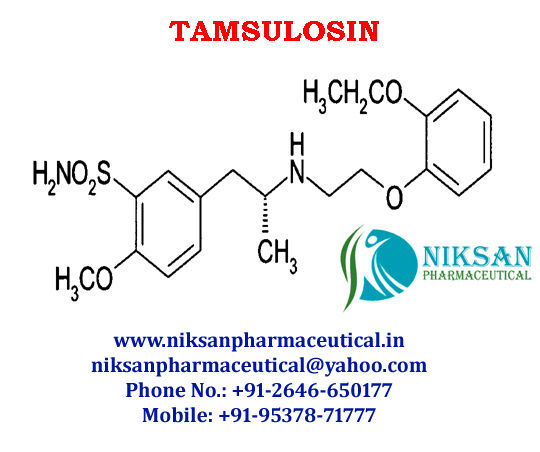
- C20H28N2O5S
- Room Temperature
- TAMSULOSIN
TAMSULOSIN Trade Information
- SAHAR AIR CARGO
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Dadra and Nagar Haveli Manipur Arunachal Pradesh Andaman and Nicobar Islands Daman and Diu Lakshadweep Uttar Pradesh Sikkim Goa North India Delhi Central India Kerala Bihar Jammu and Kashmir Telangana Andhra Pradesh Chandigarh Rajasthan Gujarat Punjab Tripura Madhya Pradesh South India Mizoram Jharkhand Tamil Nadu Odisha Assam Uttarakhand East India Meghalaya Pondicherry West Bengal Nagaland Maharashtra Haryana Karnataka West India Chhattisgarh Himachal Pradesh All India
- FDCA, GMP, GLP AND ISO
Product Description
Tamsulosin belongs to the alpha-blocker group of medicines. Tamsulosin relaxes the bladder muscles so that urine can flow easily. Tamsulosin is used to treat enlarged prostates (BPH) in men. It should be stored in a dry place at room temperature, away from direct sunlight and heat. Do not store it in the bathroom or kitchen. Read the instructions on the label and consult a doctor if you have any questions. Take your medication 30 minutes before a meal. Never open, crush, or chew the capsule. Regular use improves results.
comes under the alpha-blocker medicine. Tamsulosin relax the muscles of bladder and by this the urine can flow easily.
Tamsulosin used in the treatment of enlarged prostate (BPH)(benign prostatic hyperplasia) in male.
SYNONYMS: Tamsulosin, Tamsulosina, Tamsulosine,Tamsulosinum.
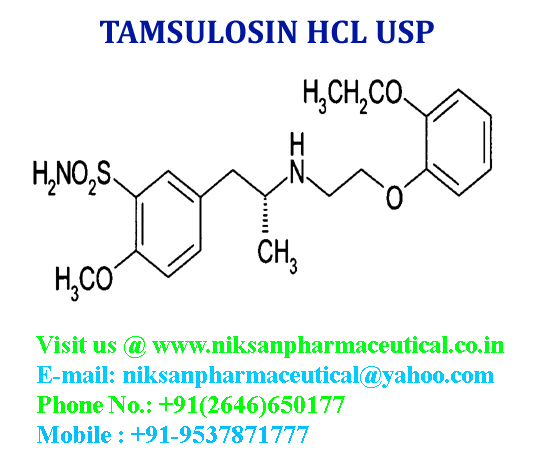
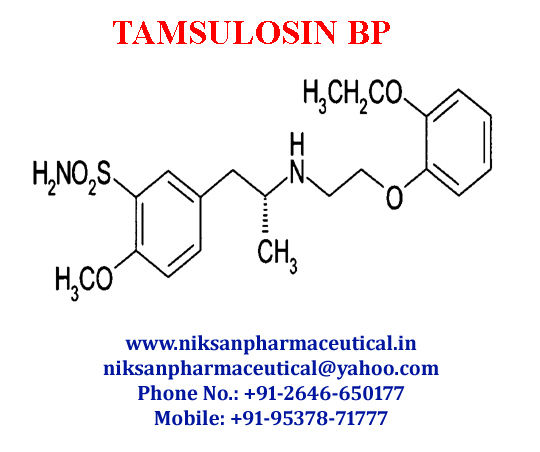
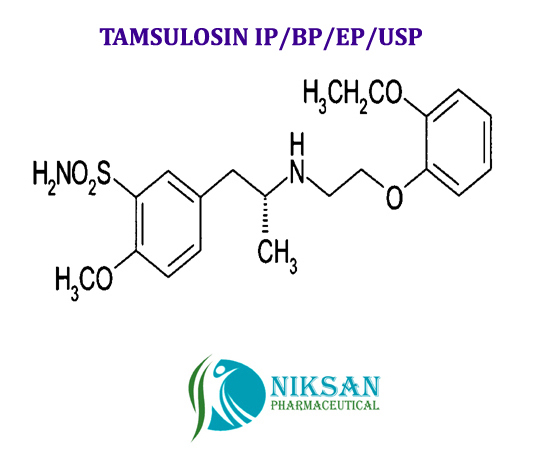
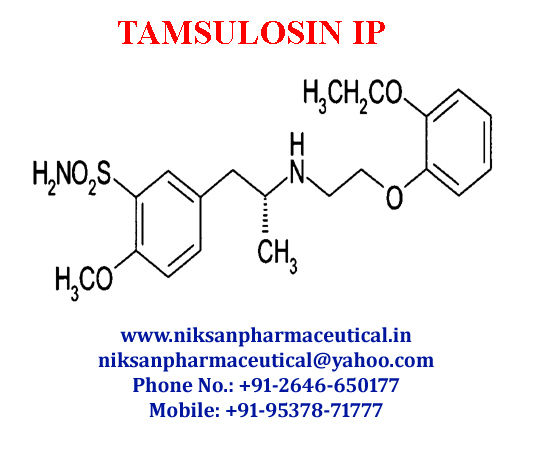
IUPACNAME: (R)-5-(2-{[2-(2-Ethoxyphenoxy) ethyl] amino} propyl)-2-methoxybenzene-1-sulfonamide
CAS NO: 106133-20-4
FORMULA: C20H28N2O5S
MOLECULAR MASS: 408.5117g/mol
STORAGE CONDITION: Store in room temperature in dry place. Put it away of light and direct heat. Do not put it in bathroom or in kitchen.
HOW TO USE: Kindly read the instruction given on the label and ask doctor for the advice. Take medication once a 30 min before the meal. Do not open, crush or chew the capsule. Take medication regularly to improve the effects.
HOW TAMSULOSIN WORKS: Tamsulosin blocks the alpha A1 and alpha 1D receptors.
By blocking the receptor the smooth muscles of kidney and bladder relaxes and by this the urinary flow improved and also prevents the storage process of bladder.
PHARMACOKINETICS OF TAMSULOSIN: Tamsulosin blocks the alpha A1 and alpha 1D receptors and relaxes the smooth muscles. The half-life of Tamsulosin is 9-13 hours.Approximately 94-99% of Tamsulosin binds with the plasma protein. 76% of drug is eliminated by the urination and the remaining drug eliminated in feces.
SIDE EFFECT OF TAMSULOSIN: The side effects of Tamsulosin are runny nose, drowsiness, ejaculation problem. If you find some serious side effect like fainting, abdominal pain, pain in kidney or breathing problem contact you doctor.
PRECAUTION: Tell your doctor if you have problems like low blood pressure, glaucoma and some eye problems. If you have allergy from Tamsulosin, kindly take alternative medication. Tamsulosin cause low blood pressure and also cause fainting which increases the risk of falling. So kindly take care about it. Do not do any exercise or heavy lifting.
CDSCO APPROVAL: Tamsulosin +Finasteride approved by CDSCO in India in 19.03.2004,
Tamsulosin + Tolterodine (0.4mg MR Pellets +2mg/4mg ER Pellets) Capsules approved by CDSCO in India in 17.11.2008,
Combikit of Tamsulosin HCl 0.4mg M.R capsules(10 no.) & Dutasteride Soft Gelatine Capsule 0.5mg (10no.) approved byCDSCO in India in 19.01.2006,
Tamsulosin HCl MR Capsules approved by CDSCOin India in 04.04.2002,
Tamsulosin HCl (MR) 0.4mg + Dutasteride 0.5mg tablets approved by CDSCO in India in 06.02.2006,
Tolterodine Tartrate 4mg (ER) + Tamsulosin HCL 0.4mg Capsules approved by CDSCO in India in 15.06.2007.
FORMULATIONS AVAILABLE IN MARKET:
Tamsulosin 0.2mg pellets
Tamsulosin 0.4 mg pellets
Tamsulosin + Finasteride capsules
Tamsulosin 0.4mg + Tolterodine 2mg pellets
Tamsulosin 0.4mg + Tolterodine 4mg pellets
Tamsulosin HCl 0.4mg M.R&Dutasteride Soft Gelatine Capsule 0.5mg
Tamsulosin HCl (MR) 0.4mg +Dutasteride 0.5mg tablets
TolterodineTartrate 4mg (ER) + Tamsulosin HCL 0.4mg Capsules
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese