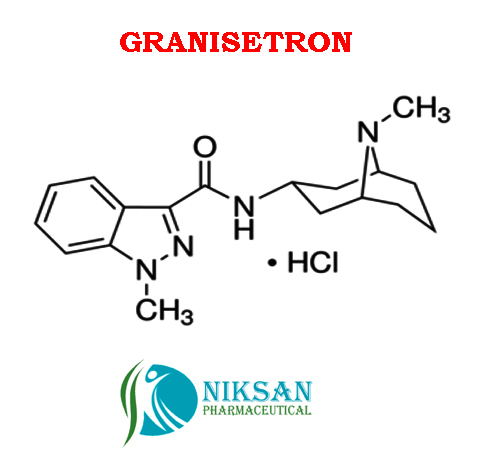
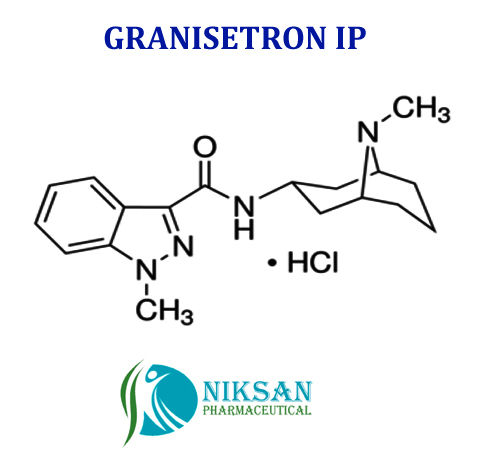
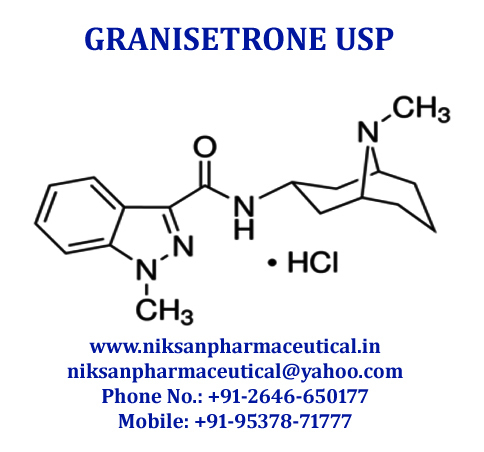
GRANISETRON
Product Details:
- Smell Resinous
- Storage Room Temperature
- Molecular Weight 312.41 Grams (g)
- Taste Odorless
- HS Code 29420090
- Molecular Formula C18H24N4O
- Shelf Life 3 Years
- Click to View more
GRANISETRON Price And Quantity
- 100 Gram
- 600 INR/Gram
GRANISETRON Product Specifications
- 3 Years
- C18H24N4O
- Powder
- GRANISETRON
- 29420090
- Other
- Medicine Grade
- GRANISETRON
- 109889-09-0
- Odorless
- Granisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors.
- Room Temperature
- 312.41 Grams (g)
- 99.8 %
- White crystalline powder
- Resinous
GRANISETRON Trade Information
- Navaseva
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Gram Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Dadra and Nagar Haveli Himachal Pradesh Pondicherry Nagaland Uttarakhand Daman and Diu Lakshadweep Sikkim Mizoram Karnataka East India Gujarat Goa Rajasthan Jammu and Kashmir Delhi Manipur Jharkhand Arunachal Pradesh Meghalaya North India Assam Tripura Telangana Bihar Punjab Chandigarh Central India South India Maharashtra Andaman and Nicobar Islands Tamil Nadu Uttar Pradesh Haryana West Bengal Madhya Pradesh Andhra Pradesh Kerala Odisha West India Chhattisgarh All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical and our group companies Niksanhealthcare are the one of largest manufacturer and supplier of Granisetron formulations and Granisetron API in Ankleshwar, Gujarat,India. We are sullying the best quality of Granisetron API allaround the India as well as in the whole world. Our product Granisetron praised by our clients and alsoby the other companies.
Niksan Pharmaceutical exporting very big quantity of the finesquality products of Granisetron inall over world for many years in acountries like Romania, Finland, Malaysia, Netherland, South Africa, Brazil,Egypt, Singapore, Jordan, Lebanon, Israel, Vietnam, Bangladesh, Paraguay,Argentina, Dominican Republic, Sudan, Hong Kong, Seychelles, Algeria,Iran,Uruguay, Russia, Thailand, Afghanistan, Latvia, Lithuania, United ArabEmirates, Seychelles, Peru, Switzerland, Tunisia, France,Hungary, Finland,Turkey, Pakistan, Rwanda, South Africa, Denmark, Malawi, Croatia, Slovenia,Ireland, Zambia, Cyprus, Nigeria, Uzbekistan, Cameroon, Netherlands,Azerbaijan, Venezuela, Morocco, Cote D Ivoire, St Lucia, South Korea, Congo,Philippines, Colombia Sweden, Hungary, Mauritius, Vanuatu, Malta, Kazakistan,Slovenia, Bolivia, Japan, Uganda, Australia and many more countries.
Niksan Pharmaceutical also supply large quantity of Granisetron products in Indian stateslike Gujarat, Haryana, Rajasthan, Madhya Pradesh, Kerala, Tamilnadu, Delhi,Bihar, Uttar Pradesh, Assam, Goa, Hyderabad, Telangana, Mizoram, Sikkim etc.
Granisetron belongs to the class of 5-HT3 receptor antagonist. Granisetron prevents nausea and vomiting by serotonin which is a natural substance which can cause nausea and vomiting.
Granisetron used as an antiemetic agent to treat vomiting and nausea in the treatment like chemotherapy. This drug did not have effect in motion sickness and it will not effect on dopamine receptors.
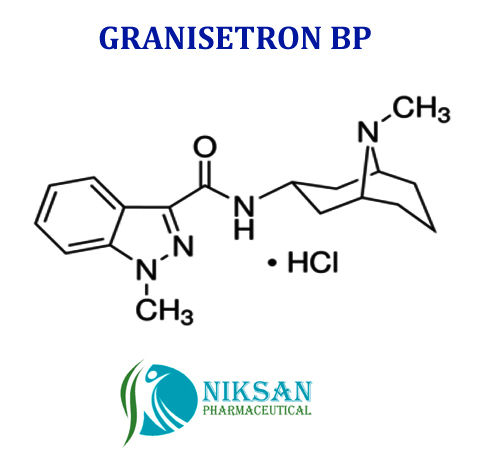
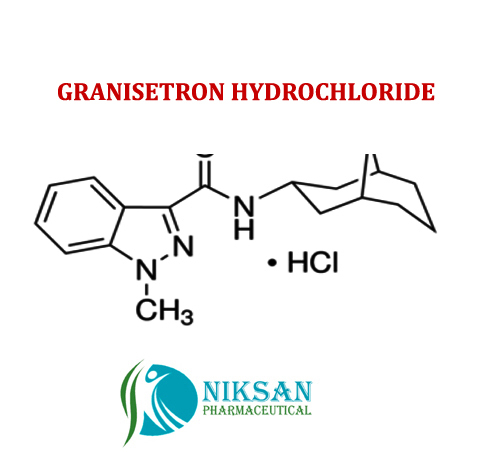
SYNONYMS OF GRANISETRON: Granisetron,Granisetronum
IUPAC NAME: 1-Methyl-N-((1R, 3r, 5S)-9-methyl-9-azabicyclononan-3-yl)-1H-indazole-3-carboxamide
CAS NO: 109889-09-0
MOLECULAR FORMULA: C18H24N4O
MOLECULAR MASS: 312.41g/mol
STORAGE OF GRANISETRON: Keep medication is cool and dry place, do not store in bathroom or kitchen. Keep all medication away from children and pets
HOW TO USE: Granisetron comes in oral dosage form like tablet; it is consumed by mouth with water. Take the medicine 1hr before the chemotherapy. And take second dose after the 12hr interval of first dose.
HOW GRANISETRON WORKS: Granisetron gives antiemetic activity by inhibiting the 5-HT3 receptor which are located in centrally and peripherally in the body. By this process the natural serotonin secretion inhibited and by this the nausea and vomiting is prevented.
PHARMACOKINETICS OF GRANISETRON: The main absorption of Granisetron is done by the oral and it is rapid and complete. As the result of first pass metabolism the oral bioavaibility is reduced about 60%. The half-life of Granisetron in the cancer patients is 9-12hrs and in the normal patient the half-life is 4-6 hrs. The 48% excretion of Granisetron is done by urination and remaining will excreted in faces.
SIDE EFFECTS OF GRANISETRON: There are some common side effects of Granisetron is headache, dizziness, fever, pain,redness and swelling on the injection point. Tell your doctor if you see side effects like stomach/abdominal pain, chest pain, fast irregular heartbeats,hallucination, loss of coordination, muscle pain etc. The allergic reactions by the Granisetron are rare but if you develop symptoms like rash, itching or irritation consult your doctor.
PRECAUTIONS: Tell your doctor if you are allergic to Granisetron or its residues. Before using the Granisetron tell your doctor if you have any past related to stomach or intestinal problem like swelling or surgery. Tell your doctor if you have certain history related to heart problems. Talk to your doctor about taking Granisetron safely.
CDSCO APPROVAL OF GRANISETRON: Granisetron tablets are approved by CDSCO in India in 05.07.2000
FORMULATIONS AVAILABLE IN MARKET:
Granisetron 1mg tablets
Granisetron 1mg/ml injection
Granisetron 2mgtablets
Granisetron 1mg/5ml injection
Granisetron 3mg/3ml injection
Granisetron 500mg tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese